Clinical Skills
Discover guidance for assessing patients, diagnostic testing, and surgical procedures through expert-led articles and videos. Perfect for general practitioners, veterinary students, and residents looking to refine their skills.

Low body temperature is associated with increased risk of hospitalization and mortality in humans. This retrospective study asked if the same holds true for cats presented for emergency care by examining the relationship between body temperature at admission and survival.
Topics In Clinical Skills
 SPONSORED
SPONSOREDClinical Assets from Antech
Our clinical skills are the bread and butter of our ability to provide high-quality medicine for our patients. Explore more resources from Antech Diagnostics to boost your clinical prowess—from wellness diagnostics to parasite detection.
Featured in Clinical Skills
New in Clinical Skills
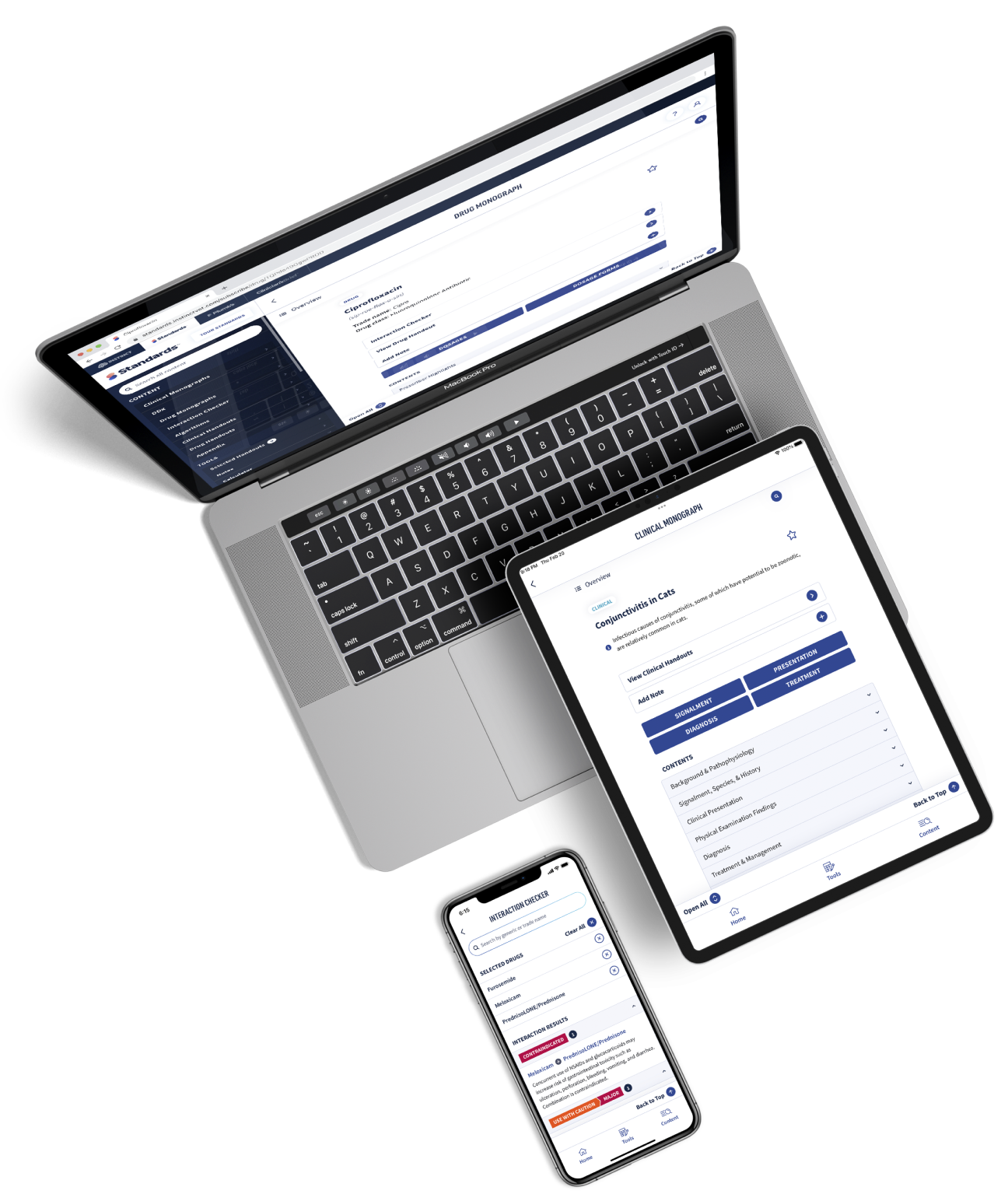
Get more clinical guidance with Standards of Care™
From the team that brings you Clinician’s Brief, extend your knowledge with expert-written, peer-reviewed diagnostic and treatment guidance as well as pet owner education and all the reliable drug information you love in Plumb’s.