Clinical Skills
Discover guidance for assessing patients, diagnostic testing, and surgical procedures through expert-led articles and videos. Perfect for general practitioners, veterinary students, and residents looking to refine their skills.
When insulin adjustments fail to achieve glycemic control, something else is usually driving the problem. Follow this case to see how comorbidities and daily management challenges contributed to poor diabetes regulation.
Topics In Clinical Skills
Poll
Brought to You by Elanco

Quiz
Brought to You by Elanco

New in Clinical Skills
Sponsored byArthrex Vet Systems
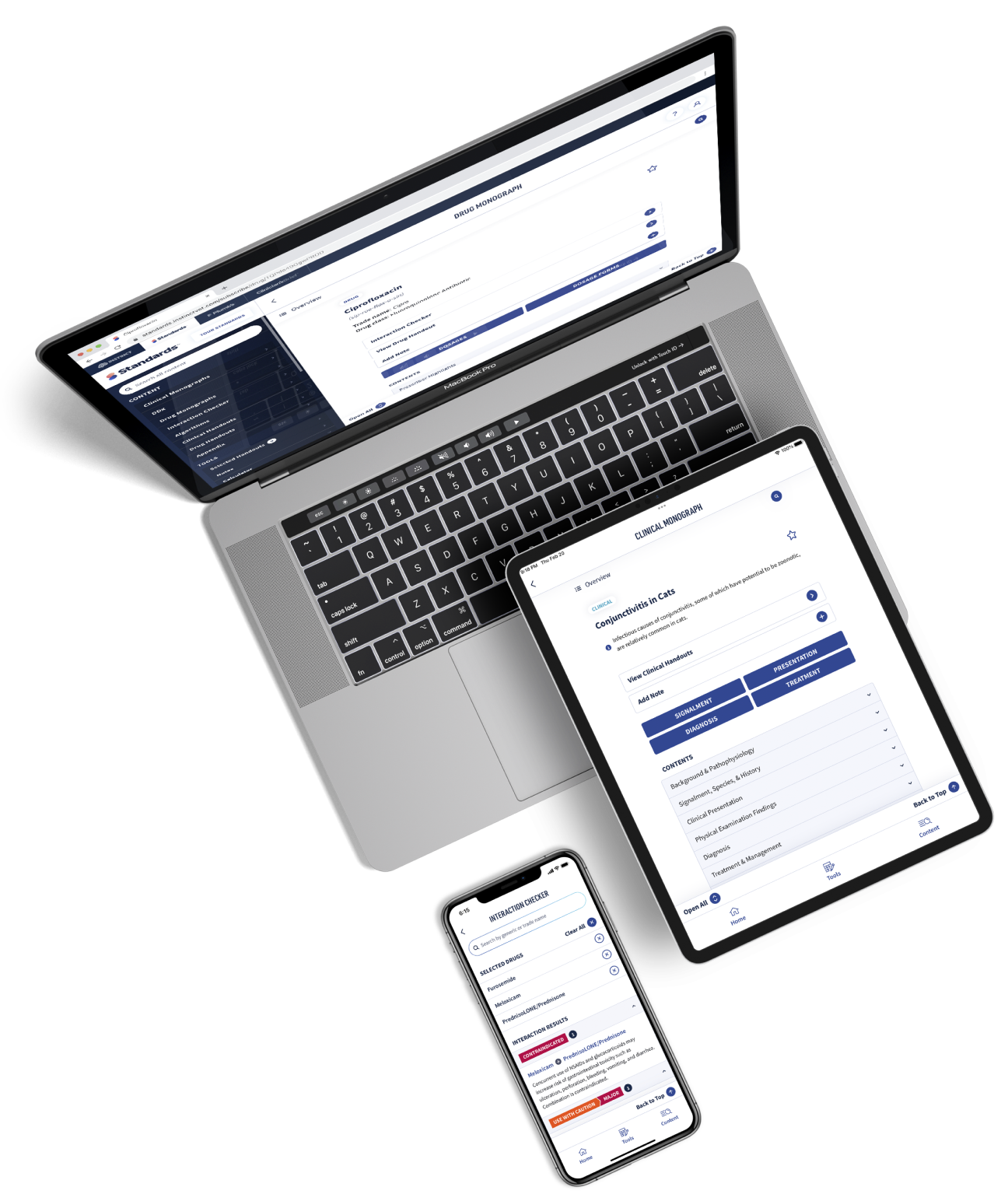
Get more clinical guidance with Standards of Care™
From the team that brings you Clinician’s Brief, extend your knowledge with expert-written, peer-reviewed diagnostic and treatment guidance as well as pet owner education and all the reliable drug information you love in Plumb’s.