Drugs & Therapeutics
Learn about the latest veterinary medications and treatment options, including dosages, side effects, and interactions, from experts in the field.
Sponsored By 

What do you do when medication alone doesn’t resolve a behavior concern? This case-based quiz outlines practical next steps for managing feline anxiety, fear, and aggression beyond fluoxetine.
Topics In Drugs & Therapeutics
New in Drugs & Therapeutics
Sponsored byPRN® Pharmacal
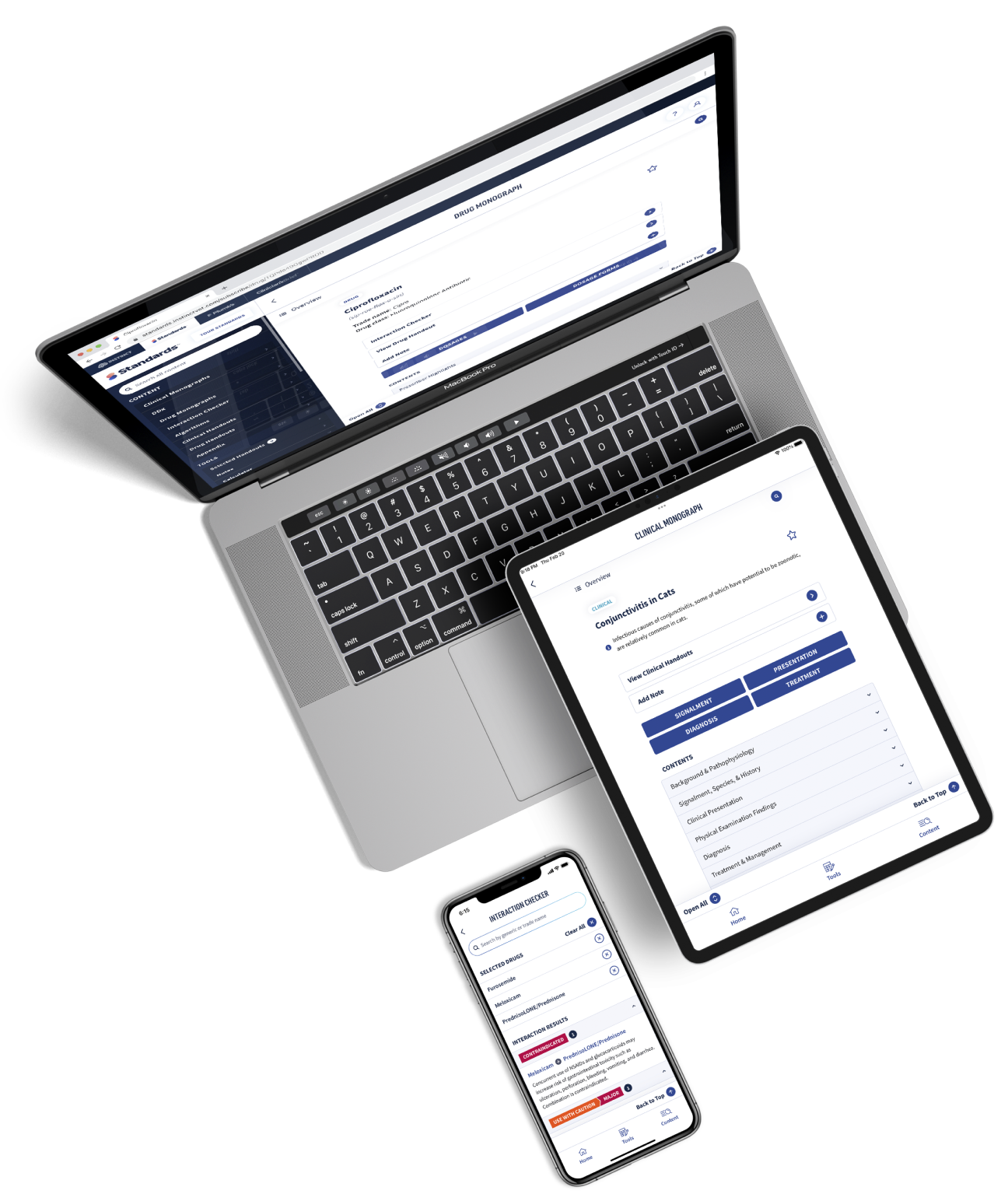
Get more clinical guidance with Standards of Care™
From the team that brings you Clinician’s Brief, extend your knowledge with expert-written, peer-reviewed diagnostic and treatment guidance as well as pet owner education and all the reliable drug information you love in Plumb’s.