Practice Life
Explore practical perspectives from peers and experts for navigating the social and professional challenges veterinarians face in the practice and beyond.
As new federal student loan caps are set to take effect in 2026, the latest AVMA data show average veterinary student debt—and debt-to-income ratios—are rising once again. In this episode, Dr. Alyssa and Dr. Beth explore what these financial trends could mean for long-term career sustainability, workforce access, and the future of the profession.
Topics In Practice Life
Featured in Practice Life


New in Practice Life
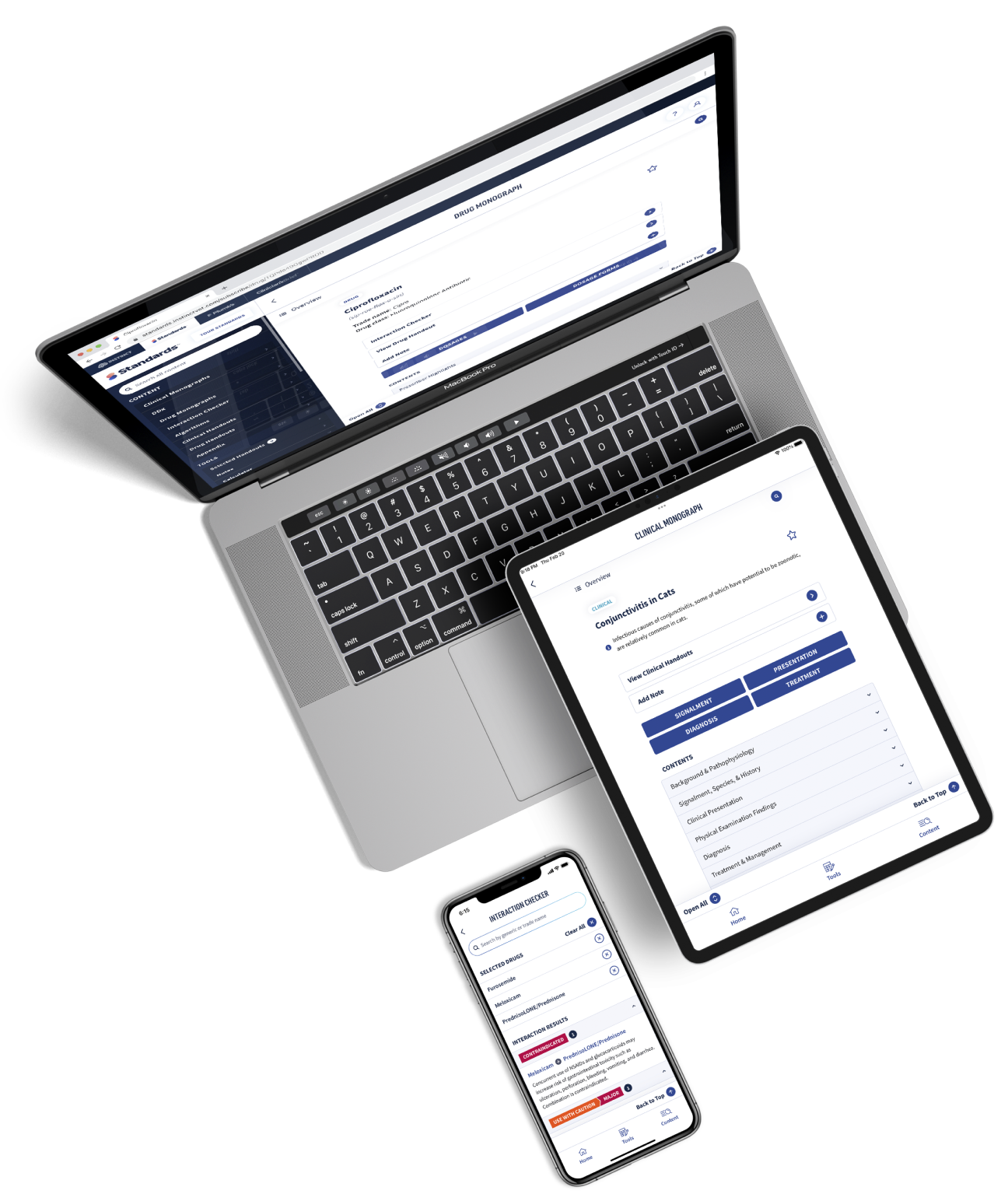
Get more clinical guidance with Standards of Care™
From the team that brings you Clinician’s Brief, extend your knowledge with expert-written, peer-reviewed diagnostic and treatment guidance as well as pet owner education and all the reliable drug information you love in Plumb’s.