Cyclosporine: Case Studies for Alternative Uses
Alexander Werner Resnick, VMD, DACVD, Animal Dermatology Center
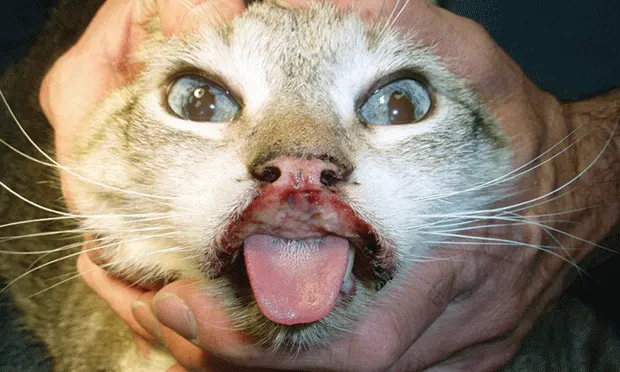
This is the second installment of a two-part series outlining alternative uses of cyclosporine. The first installment discussed the pharmacology, dosing, and monitoring of cyclosporine (CsA); the following describes three dermatologic conditions with related studies supporting treatment with CsA.
Related Article: Cyclosporine: An Overview for Alternative Use
Sebaceous Adenitis
Sebaceous adenitis (SA), an inflammatory disorder of undetermined cause, likely results from the immune-mediated destruction of sebaceous glands and anatomic or cornification defect of the sebaceous duct (causing inflammatory reaction). Autosomal recessive inheritance has been proposed for the standard poodle and Akita; however, the disease has been identified in most breeds. SA is rare in cats. Because of increased antigen-presenting cells in the inflammatory infiltrate associated with gland destruction, CsA may be effective in controlling disease progression.
SA has several distinct presentations, but the hallmark clinical sign is adherent crusting of hair shafts with follicular casts, commonly referred to as keratin collaring. Over time, the hair coat becomes brittle and thin. SA may be classified into 2 types: long-coated breeds and short-coated breeds.
Figure 1. Keratin collaring of hair shafts on patient with SA.

In long-coated breeds, lesions initially develop on the dorsal muzzle and temporal region, eventually progressing to include the body (dorsally and bilaterally symmetrical) and tail. The most commonly affected of the long-coated breeds are the standard poodle and the Akita; German shepherd dogs, Samoyeds, and Havanese are also reportedly predisposed. In standard poodles, initial crusts may be silvery and fine before progressing to tightly adherent and thickened fronds.
The normally curly hair coat becomes thinned and straight, starting on the face/pinnae and moving dorsally to neck and trunk.
Figure 2. SA in a standard poodle; the hair coat has become straight and thin, with accumulation of scale.

Standard poodles may develop subclinical disease, diagnosed by histopathologic examination of skin biopsy samples. It is currently unknown whether some or all patients with abnormal biopsy findings will develop clinical disease or should be treated with CsA. In the Akita, signs develop in similar areas, compared with the standard poodle, but rapidly generalize with thick, adherent custs and more severe, erythematous, greasy lesions, often leading to secondary, deep pyoderma. This can result in increased morbidity.
In short-coated breeds (eg, Vizsla, Dachshund), SA presents as discrete annular plaques with alopecia and adherent fine scale. Lesions develop primarily on the body, as well as on the face and pinnae.
Figure 3. Scales and crusts on the head of a Vizsla with SA.

These patients develop significant scarring but infrequently have concurrent pyoderma.
Multiple SA treatment options have been reported with recent focus on the use of CsA. A partly blinded, placebo-controlled study compared the use of CsA (5 mg/kg PO q24h) and topical therapy (concurrently and separately) for treating this disease and established that both treatment modalities were effective, but a synergistic effect was noted with concurrent administration.1 In particular, CsA-treated groups (alone or with topical therapy) produced a significant increase in the number of sebaceous glands found in tissue samples after 4 months of therapy.1 Significant improvements in alopecia and scaling were also reported, especially in the combined topical therapy and CsA treatment group.1
Related Article: Feline Pemphigus Foliaceus: A Common Autoimmune Dermatosis
Perianal Fistulae
Also known as anal furunculosis, canine perianal fistula (PAF) is a progressive inflammatory disease producing cutaneous as well as rectal fistulae with ulceration findings somewhat similar to those of Crohn’s disease in humans. German shepherd dogs are predisposed. The cause is unknown, but immunohistochemical analysis of tissues from dogs with PAF supports an immune-mediated cause, likely from T-helper cell dysregulation.
Clinical signs of PAF can include perianal licking, tail tenderness, changes in tail carriage, constipation, tenesmus, dyschezia, and mucopurulent discharge from the anal area.
Figure 4. Perianal fistulae in a German shepherd dog.

Previous management of PAF (eg, immunosuppression and surgical debridement of affected tissues) has resulted in inadequate or temporary relief.
Successful treatment of PAF with CsA has been reported in several studies2,3 in which the effective dosage of CsA ranged from 2 mg/kg PO q24h to 10 mg/kg PO q12h. Concomitant administration of ketoconazole to reduce CsA dosage can minimize cost with similar efficacy. Current guidelines recommend treating PAF with CsA (5–7.5 mg/kg PO q24h) and ketoconazole (5–10 mg/kg PO q24h).2,3 Once lesions resolve, PAF can be controlled via continued q24h to q48h administration: few cases resolve completely, and daily or alternate daily administration at lower-than-induction dosages of both medications may be required to prevent relapse. Tacrolimus, a topical macrolide that can be used in conjunction with CsA, has also demonstrated efficacy in the treatment and management of PAF.4
Unlike treatment of atopy and SA, when treating PAF serum trough level measurement of CsA can help determine dosage requirements; the measurement should remain between 100 and 300 ng/mL for maintenance (depending on the reference range for the laboratory).5
Feline Eosinophilic Skin Disease
Commonly referred to as feline eosinophilic granuloma complex, feline eosinophilic skin disease (FESD) is a term for 4 distinct syndromes: eosinophilic plaque, eosinophilic granuloma, indolent ulcer, and allergic miliary dermatitis. FESD lesions are grouped primarily because of clinical similarities, frequent concurrent (and recurrent) development, and positive response to corticosteroids. A genetic predisposition to develop lesions has been documented, but multiple studies have confirmed that the majority of lesions have allergic causes. Each syndrome remains distinct in presentation.
Eosinophilic Plaques
Eosinophilic plaques are alopecic and eroded, well-demarcated annular patches and steep-walled plaques with a glistening or exudative surface and usually occur in the inguinal, perineal, lateral thigh, and axillary regions.
Figure 5. Eosinophilic plaques on the ventrum of a DSH cat.

Eosinophilic Granulomas
Eosinophilic granulomas have 5 occasionally overlapping presentations: linear granulomas (ie, individual to coalescing plaques arranged in a linear pattern on the caudal thigh), nonlinear granulomas (ie, individual to coalescing plaques anywhere on the body with an ulcerated and white or yellow cobblestone appearance), asymptomatic lip margin and chin swelling (eg, pouting), footpad swelling (including metacarpal and metatarsal) with pain and lameness, and oral granulomas (ie, ulcerated single to coalescing plaques on the tongue, palate, and palatine arches with a white/yellow cobblestone appearance).
Figure 6. Pouty chin of eosinophilic granuloma.

Indolent Ulcers
Indolent ulcers are large, concave, well-demarcated and indurated, often asymptomatic lesions confined to the upper lips adjacent to the philtrum.
Figure 7. Indolent ulcer affecting the entire upper lip.

Allergic Miliary Dermatitis
Allergic miliary dermatitis is commonly seen as multiple black/brown crusted and erythematous papules. Lesions are more often palpated than visualized and associated with moderate to severe pruritus.
Related Article: Canine Atopic Dermatitis
Several case reports and retrospective and prospective studies have evaluated CsA for treating FESD lesions.6,7 Excellent response has been reported with dosages between 5 and 12.5 mg/kg q24h.6,7 Although administration of CsA to FESD patients can be successfully reduced to q48h, a higher dosage (7.5 mg/kg) than administered for uncomplicated atopy is often necessary to maintain adequate control of lesions.
Because of increased wandering and hunting behaviors, cats have more potential for pathogen exposure than dogs, making immunosuppressive effects of CsA at higher dosages concerning, especially with concurrent glucocorticoid administration.8,9 In one study, 25% of renal transplantation cats receiving CsA developed an infectious disease complication.8 Appropriate guidelines for the use of CsA in cats include housing cats indoors, reducing parasite exposure (flea/tick control), feeding cooked or commercial food, monitoring blood trough CsA to maintain levels below 400 ng/mL, and obtaining pretreatment blood work with negative FeLV and FIV and Toxoplasma spp titers (if patient has outdoor exposure).
Conclusion
The list of diseases currently treated with CsA is rapidly expanding. CsA is a potent medication; like glucocorticoids, it has dosage-dependent immunomodulatory and immunosuppressive activities. CsA has a specific effect on the activation of T-cells and dendritic cells, as well as more general effects on inflammatory cells and keratinocytes. This makes it ideal for many dermatologic conditions. However, it is not a substitute for proper diagnosis and should not be prescribed without diligence.
CsA is often prescribed with other medications and topical therapy to minimize dosage and (therefore) potential for adverse events. The bioavailability and patient tolerance of CsA can vary widely. Appropriate monitoring (ie, routine blood counts, serum chemistries, urinalysis with culture) with CsA, as with all medications, is necessary. Over time, the dose of CsA may be reduced or even discontinued while still maintaining control.