Cyclosporine: An Overview for Alternative Use
This review, the first in a two-part series, explores the mechanics of cyclosporine in off-label treatment. The second installment will describe its use in specific disease case studies.
Cyclosporine (CsA) is a cyclic polypeptide immunosuppressant produced by the fungus Beauveria nivea. In 2003, the microemulsion formulation (Atopica) was FDA-approved to help control atopic dermatitis in dogs weighing a minimum of 4 lb. Atopica has been released in liquid form for cats and small dogs.
Related Article: Canine Atopic Dermatitis
MechanicsCsA inactivates calcineurin phosphorylase in T cells, preventing transcription of interleukin-2 (IL-2) as well as other cytokines. IL-2, required for the activation of the T-cell pathway, specifically affects the T-helper cells (Th1, Th2). As a result, CsA administration inhibits activation of T cells, natural killer cells, and Langerhans (ie, antigen-presenting) cells; it also depletes dermal and epidermal lymphocytes and macrophages. Suppression of the Th1 or Th2 response induces antigen tolerance.1
Additional studies report that CsA reduces keratinocyte cytokine secretion and hyperproliferation, histamine release from mast cells, and growth and differentiation of B cells. Despite these and other potent immunomodulatory effects, studies have failed to detect humoral immune response suppression as well as alteration of allergen-specific IgE serology or intradermal testing with house dust mite and flea antigens.2
As CsA is a highly lipophilic molecule that is poorly absorbed when taken orally, it should not be prescribed unmodified (vegetable oil formulation). The modified form, an increased hydrophilic microemulsion (ME), increases bioavailability from about 22% to 35% and decreases individual variability in absorption.1 The following discussion assumes the use of the veterinary ME CsA formulation (ie, Atopica). The pharmacokinetics of generic CsA formulations vary, making it important to monitor patients closely when switching brands. The author has witnessed treatment failures when replacing the name brand with generic formulas.
For optimum absorption, CsA should be administered in a single, daily dose and not within 2 hours of a meal, although the prolonged effect of administration with food may not be significant.3 CsA widely distributes in the body and binds predominately to plasma lipoproteins. Concentration in skin can be up to 10 times that found in blood.4 Systemic clearance is regulated primarily by cytochrome P450 isoenzymes in the liver; therefore, drug elimination can be modified via medications that alter or compete with this pathway (eg, azole antifungals).
Related Article: Five Questions for a Dermatologist
Indications & Advantages

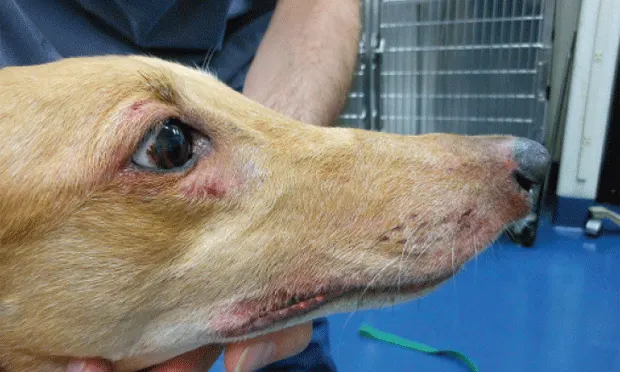
Atopica has been approved (and most used) for treating canine and feline atopic dermatitis (Figures 1 and 2). A systematic review and metaanalysis of CsA efficacy for the treatment of atopic dermatitis reported equal efficacy as compared with glucocorticoids.5 CsA is under evaluation for treatment of various dermatoses affecting dogs and cats and may be considered for many glucocorticoid-responsive disorders.6
Figure 1. 7-year-old spayed greyhound with atopic dermatitis. Note the erythema and excoriation of periocular and lip regions.
Figure 2. Patient in Figure 1 with severe erythema and excoriation of the plantar surface of the foot.

Standard dosage is 5 mg/kg q24h for dogs and 7 mg/kg q24h for cats. Patients that experience GI distress may need the initial dose decreased by 50% for 15 days and then increased to full dose to permit acclimation to medication. Alternatively, anti-emetic drugs can be administered during the induction period (metoclopramide at 0.2–0.4 mg/kg q12h or maropitant citrate at 2 mg/kg q24h for the first 4 days of each dosage change). Metoclopramide may increase absorption of CsA by increasing gastric emptying; however, the author has not recognized negative clinical effects at the dosages used to treat dermatologic conditions in dogs. Most patients demonstrate a noticeable reduction (>30%, per author) in signs within 4 weeks, although maximum improvement may not be seen for 16 weeks.
Once sufficient response (subjectively determined by owner and veterinarian) is achieved, administration may be decreased to q48h at full dose. Some patients may have their dose interval temporarily increased or even stopped while still maintaining adequate disease control. Patients with persistent GI difficulties may tolerate and respond to lower, more frequent doses. Those that tolerate the medication but do not achieve sufficient response may have the dosage of CsA increased, but the author recommends monitoring CsA blood trough concentration in these cases.
Contraindications & DisadvantagesDespite variables among different patients and drug brands (ie, drug absorption, metabolism, effect), monitoring serum or whole blood drug levels is not required when determining the necessary CsA dosage for optimum advantage in most canine patients. CsA blood trough concentration can be monitored in patients with higher-than-standard doses, patients experiencing persistent adverse effects, or cats (particularly during initial treatment). Because commercial laboratories differ in reference ranges and whether they test serum or whole blood, serial samples should be sent to the same laboratory for consistency.
Adverse reactions to CsA include GI distress (eg, anorexia, vomiting, diarrhea) during induction but usually subside within several weeks. Less common reactions in dogs include hypertrichosis, gingival hyperplasia, and aberrant reactions, possibly from bacterial infection (eg, papillomatosis, psoriasiform-lichenoid–like dermatitis), which can be minimized with appropriate antibiotic administration and reduction of CsA dosage. Uncommon adverse events include increased pruritus following administration in dogs and increased incidence of upper respiratory infections and fatal toxoplasmosis in cats.
CsA is not recommended for patients with FeLV, FIV, or a history of malignancy. Reactivation of latent toxoplasmosis by CsA has been reported in rare transplantation cases. Elevated serum glucose, urea nitrogen, and creatinine levels have been noted in cats receiving CsA; therefore, routine monitoring of serum biochemistry panels is recommended.7 A retrospective study of the frequency of urinary tract infections in dogs treated with CsA reported a 30% incidence of positive urinary bacterial cultures in the absence of clinical signs of urinary tract infection. Urinalysis with culture should be part of routine monitoring of patients receiving CsA.7
Patients with glucose metabolism impairments should be monitored closely, as disturbance of glucose homeostasis by CsA has been reported. In one study, serum fructosamine and plasma glucose concentrations were significantly higher, and serum insulin levels were significantly lower after CsA administration.8 The effect of CsA administration on patients predisposed to and with diabetes mellitus remains unclear, and long-term studies have not been reported. Close monitoring of plasma glucose concentration in patients with diabetes mellitus during induction CsA therapy is recommended.
CsA may interact with other drugs because of its liver and cytochrome P450 metabolism and P-glycoprotein utilization for absorption. Of note, ketoconazole coadministration has been reported to significantly reduce the effective dosage of CsA. A recent study reported no difference in skin or blood CsA concentrations among dogs receiving 5 mg/kg q24h of CsA alone or 2.5 mg/kg q24h with 2.5 mg/kg q24h ketoconazole.9
Economic ImpactEconomic factors of prescribing CsA, whether brand name (recommended) or generic, are significant. On a q48h maintenance dosage schedule, the cost of CsA at 5 mg/kg is approximately <$ per month; the financial burden of this treatment comes from its potentially lifelong administration.
It is more cost effective and medically sound for patients with atopic dermatitis to be allergy tested and to receive allergen-specific hyposensitization. The cost of CsA treatment for patients with other diseases must be discussed with the owners before therapy is initiated.
CsA is being evaluated for the treatment of various dermatoses and may be considered for any glucocorticoid-responsive disorder.6 A brief review of several veterinary resources listed nearly 30 dermatologic diseases for which CsA has been prescribed. However, as case-controlled, published studies are still limited, the general practitioner is cautioned not to prescribe CsA for dermatologic conditions without proper workup and diagnosis, a thorough discussion of indications and adverse events with owners, appropriate monitoring during treatment, and/or referral. This can be a beneficial drug, but its misuse and/or overuse is already having a negative impact on patients. The second installment will discuss primary diseases consistently reported as responding to treatment with CsA.
ALEXANDER WERNER, VMD, DACVD, is owner of Animal Dermatology Centers in Studio City and Westlake Village, California, and Reno, Nevada. In addition to clinical practice, he has presented lectures throughout North America and published numerous articles and book chapters on dermatology. Dr. Werner is editor of the latest edition of Blackwell’s Five-Minute Veterinary Consult: Small Animal Dermatology, and coauthor of the most recent clinical companion. Dr. Werner completed his dermatology residency at University of California, Davis.
CYCLOSPORINE: AN OVERVIEW FOR ALTERNATIVE USE • Alexander Werner
_References
1. Cyclosporin: A new drug in the field of canine dermatology. Guaguère E, Steffan J, Olivry T. _Vet Dermatol 15:61-74, 2004.2. Investigation of the effects of ciclosporin (Atopica) on intradermal test reactivity and allergen-specific immunoglobulin (IgE) serology in atopic dogs. Goldman C, Rosser E Jr, Petersen A, Hauptman J. Vet Dermatol 21:393-399, 2010.3. Influence of food intake on the clinical response of cyclosporin A in canine atopic dermatitis. Thelen A, Mueller RS, Linek M, et al. Vet Rec 159:854-856, 2006.4. Cyclosporin concentration in the skin following oral administration. Steffan J, Maurer M, Rohlfs A. Vet Dermatol 2003; 14:248 (Abstract).5. A systematic review and meta-analysis of the efficacy and safety of cyclosporin for the treatment of atopic dermatitis in dogs. Steffan J, Favrot C, Mueller R. Vet Dermatol 17:3-16, 2006.6. Cyclosporin A: Applications in small animal dermatology. Robson DC, Burton GG. Vet Dermatol 14:1-9, 2003.7. Frequency of urinary tract infection in dogs with inflammatory skin disorders treated with ciclosporin alone or in combination with glucocorticoid therapy: a retrospective study. Peterson AL, Torres SM, Rendahl A, Koch SN. Vet Dermatol 23:201-e43, 2012.8. Ciclosporin A therapy is associated with disturbances in glucose metabolism in dogs with atopic dermatitis. Kovalik M, Thoday KL, Handel IG, et al. Vet Dermatol 22:173-180, 2011.9. The effect of ketoconazole on whole blood and skin cyclosporine concentrations in canines. Gray L, Hillier A, Cole L, Rajala-Schultz P. Vet Derm suppl 1:13, 2012.
Suggested Reading
A multicentre placebo-controlled clinical trial on the efficacy of oral ciclosporin A in the treatment of canine idiopathic sebaceous adenitis in comparison with conventional topical treatment. Lortz J, Favrot C, Mecklenburg L, et al. Vet Dermatol 21:593-601, 2010.Adverse events in 50 cats with allergic dermatitis receiving ciclosporin. Heinrich NA, McKeever PJ, Eisenschenk MC. Vet Dermatol 22:511-520, 2011.Cyclosporine decreases skin lesions and pruritus in dogs with atopic dermatitis: A blinded randomized prednisolone-controlled trial. Olivry T, Rivierre C, Jackson HA, et al. Vet Dermatol 13:77-87, 2002.Cyclosporine and ketoconazole for the treatment of perianal fistulas in dogs. Patricelli AJ, Hardie RJ, McAnulty JE. JAVMA 220:1009-1016, 2002.Evaluation of the effect of two dose rates of cyclosporine on the severity of perianal fistulae lesions and associated clinical signs in dogs. House AK, Guitian J, Gregory SP, Hardie RJ. Vet Surg 35:543-549, 2006.Evaluation of the prevalence of infections in cats after renal transplantation:169 cases (1987-2003). Kadar E, Sykes JE, Kass PH, et al. JAVMA 227:948-953, 2005.The use of oral cyclosporin to treat feline dermatoses: A retrospective analysis of 23 cases. Vercelli A, Raviri G, Cornegliani L. Vet Dermatol 17:201-206, 2006.