A 5-year-old, 73-lb (33-kg) neutered male crossbreed dog imported from Spain was referred for rapid-onset aural discomfort of 3 days’ duration that progressed within 2 days to blistering and ulceration of the pinnae and multiple paw pads. Flea, tick, and heartworm preventives were not up to date.
Physical Examination
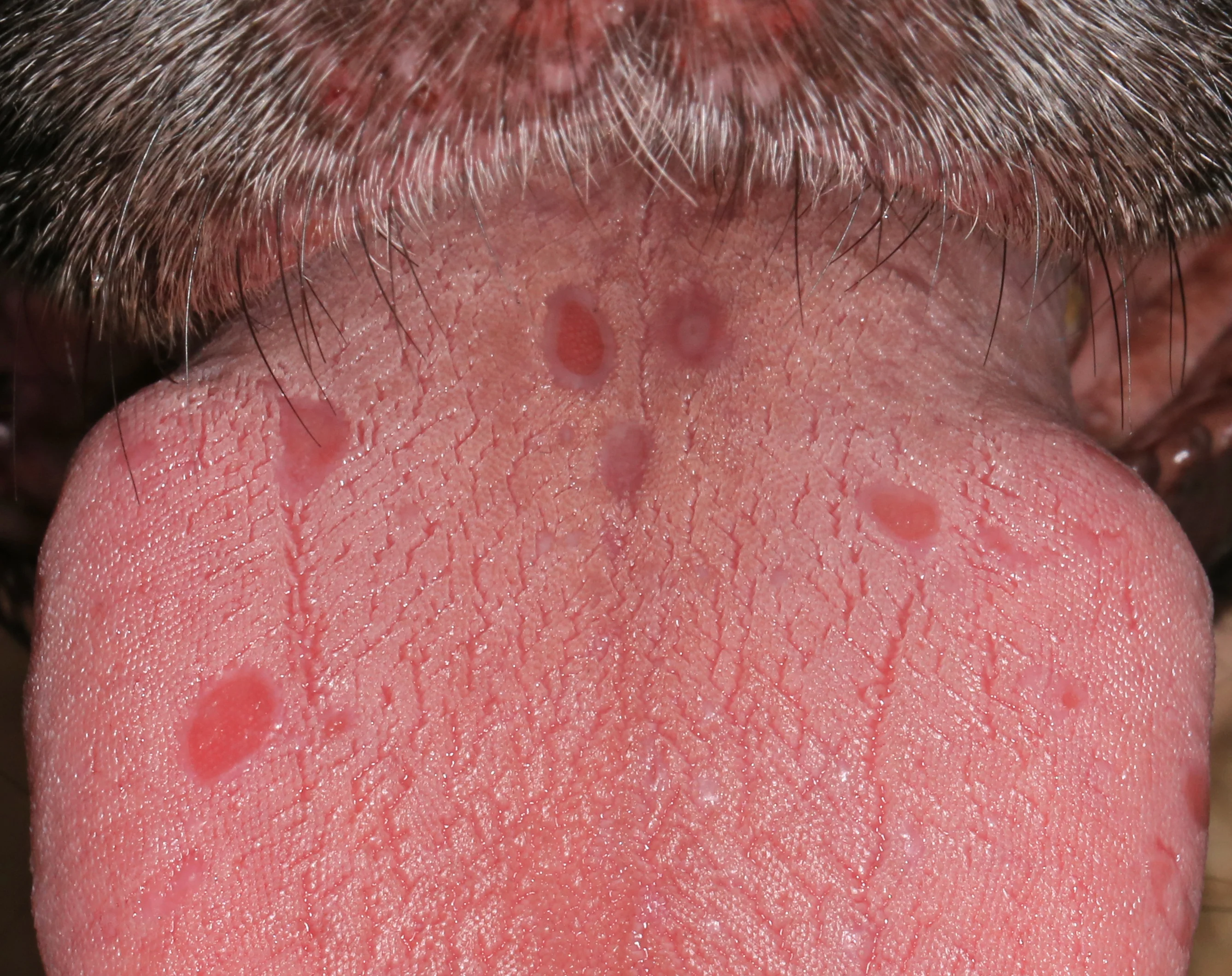
On presentation at the referral clinic, the dog was normothermic but obtunded, with nonpruritic, painful, rapidly progressing, multifocal, vesiculobullous to ulcerative dermatitis with minimal crusting. Temperature, pulse, and respiration rate were within normal limits; mucous membranes were mildly injected, but capillary refill time was unremarkable. Multiple lesions were observed, especially at the frictional sites of the skin, including the lip margins, concave pinnal surfaces and margins, inguinal region, and multiple pad margins, as well as extending onto the metatarsal paw pad surfaces and the oral mucosa (including the tongue) (Figure 1-6). Vesicles and blisters were prominent and resilient when palpated, suggesting skin cleavage was occurring deep within the dermoepidermal junction.

FIGURE 1 Vesicles on the left concave pinna
Diagnostics
On presentation at the clinic the day prior to referral, diagnostic testing was performed, including serum chemistry profile, total thyroxine, hematology, and urinalysis (including urine protein:creatinine ratio). Results were unremarkable.
At the referral clinic, point-of-care cytology obtained from intentionally ruptured vesicles, beneath pinnal crusts, within the most acute/recent paw pad margin fissures, and ulcerated lip margins showed nondegenerate neutrophils in addition to sparse, mixed, mostly free (rather than phagocytosed) bacteria within a granular eosinophilic background. No acantholytic keratinocytes were found.
Serum was submitted for immediate in-house analysis to gauge the degree of systemic inflammatory response. Results showed a high C-reactive protein level (61.1 mg/L; reference interval, 0-10 mg/L).
Differential Diagnosis
Based on signalment and clinical appearance, differentials were primarily autoimmune subepidermal blistering diseases, most likely epidermolysis bullosa acquisitawith secondary bacterial infection.1-3 Other possible conditions included pemphigus vulgaris and other autoimmune subepidermal blistering diseases (eg, bullous systemic lupus erythematosus, mucous membrane pemphigoid, bullous pemphigoid, canine linear immunoglobulin A disease), immune-mediated diseases (eg, adverse drug reaction [no recent or long-term drug exposure], erythema multiforme major/Stevens-Johnson syndrome), thermal or chemical burns, and contact irritancy (furanocoumarins in giant hogweed).
Skin swabs were submitted for microbial culture and susceptibility testing. After 5 days, matrix-assisted laser desorption ionization–time of flight mass (MALDI-TOF) spectrometry showed profuse Staphylococcus pseudintermedius with susceptibility to all tested antimicrobials.
Due to COVID-19 pandemic lockdown restrictions, there was a 2-day delay before multiple elliptical and 6- to 8-mm punch skin biopsies were collected with the patient under general anesthesia (Figure 7). The skin biopsy samples were submitted with urgent request to a dedicated dermatohistopathology team. Results showed blister formation at the dermoepidermal junction where the epidermis had separated from the underlying dermal stroma, likely through the basement membrane zone (Figure 8). Neutrophils were the predominant cell type, with no evidence of acantholytic keratinocytes, epidermal necrosis, or apoptosis.

FIGURE 7 Excised coalescing vesicles from the right pinnal margin on biopsy
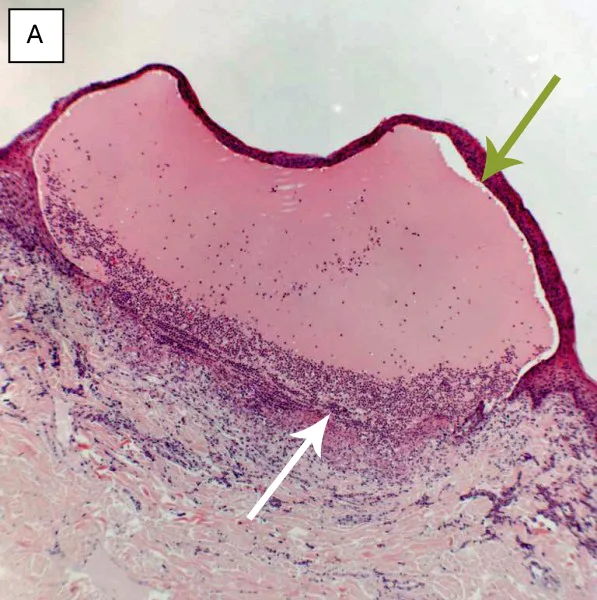
FIGURE 8 Hematoxylin and eosin–stained histopathology images showing subepidermal blistering disease—blistering at the dermoepidermal junction, where the epidermis (green arrows, stratum basale) has separated from the underlying dermal stroma (white arrows), likely through the basement membrane zone, plus a predominance of neutrophils in the superficial dermis. (A) Oral blister, magnification,×5; (B) roof of a blister, magnification,×40; (C) blister on the paw, magnification,×2.5; images courtesy of Dr. M. Silkstone, Abbey Veterinary Services.
Serum was submitted to an immunology department for immunodiagnostics. Direct immunofluorescence was delayed 6 months because of the COVID-19 pandemic; results were negative.
Additional diagnostic tests can be used to definitively diagnose epidermolysis bullosa acquisita.4,5 Circulating autoantibodies to immunoglobulin G can be detected via indirect immunofluorescence assay using salt-split canine buccal mucosal tissue (Figure 9). Autoantibodies targeting the noncollagenous domain of collagen VII can be confirmed in most tested dogs via immunoblotting (Figure 10), ELISA with human recombinant proteins, or canine noncollagenous-transfected 293 T cells.
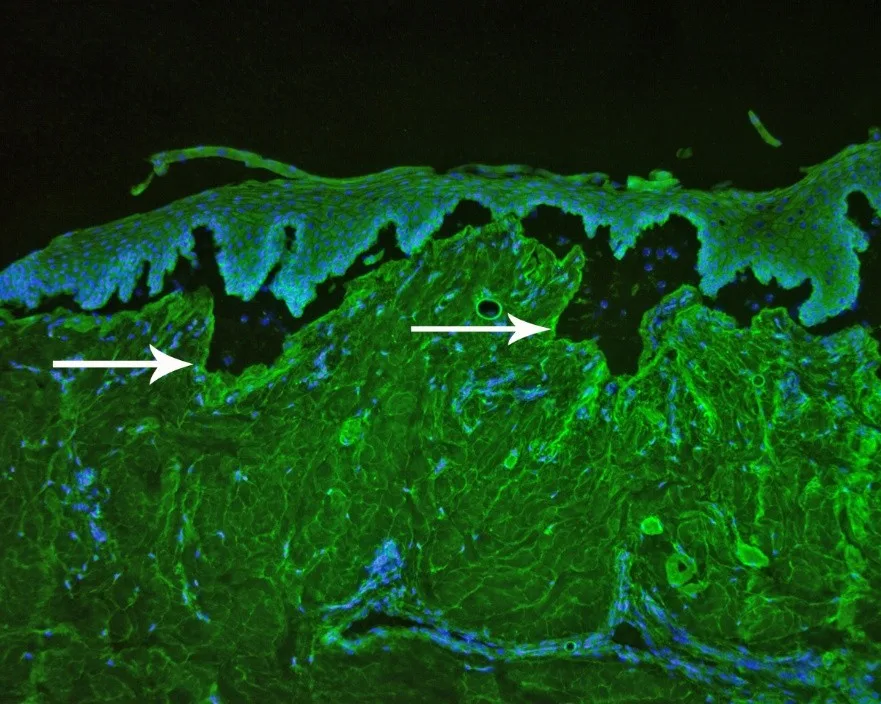
FIGURE 9 Indirect immunofluorescence assay showing immunoglobulin G autoantibodies targeting the dermal side of salt-split canine skin (arrows). Magnification ×10. Image courtesy of T. Olivry, North Carolina State University.
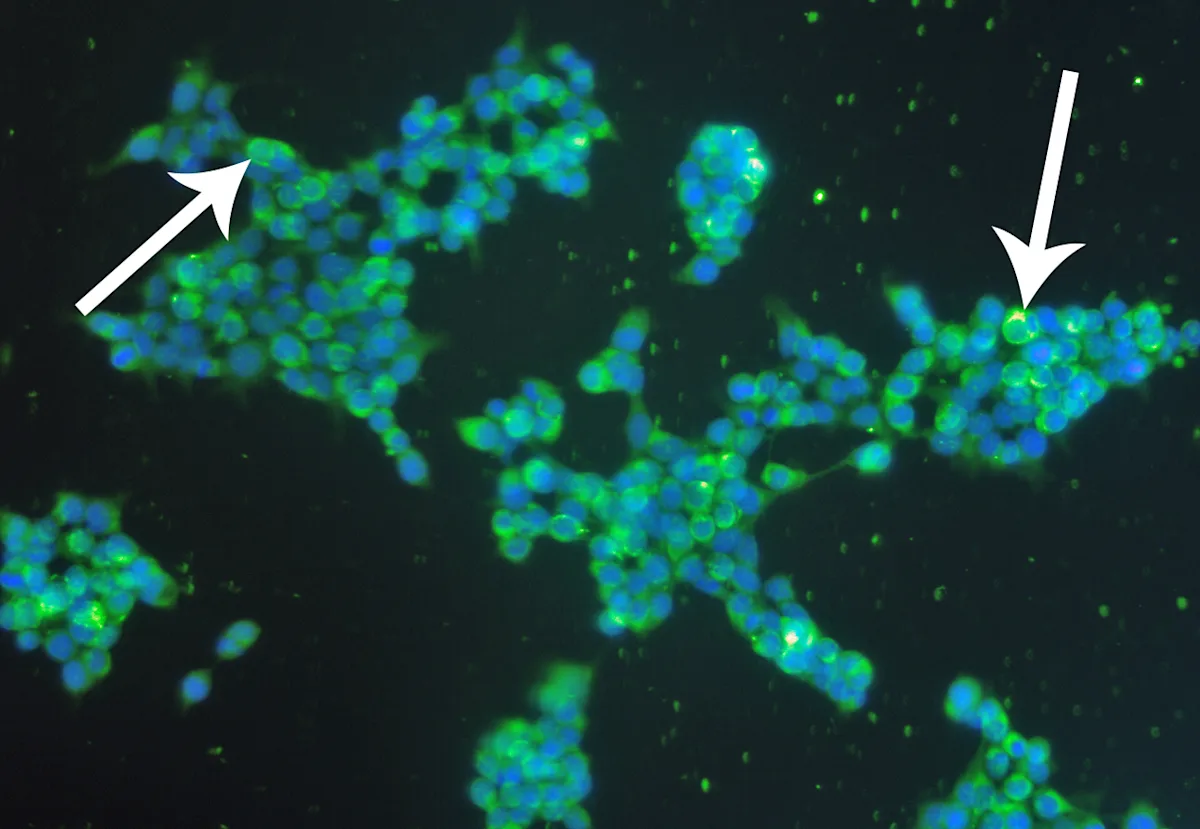
FIGURE 10 Indirect immunofluorescence examination, using human embryonic kidney cells, transfected with the aminoterminal noncollagenous domain of type VII collagen showing positive fluorescence (arrows). Magnification ×20. Image courtesy of T. Olivry, North Carolina State University.
Diagnosis: Epidermolysis Bullosa Acquisita
Diagnosis
Epidermolysis bullosa acquisita was provisionally diagnosed. Because of the patient’s rapidly deteriorating condition, an aggressive, multimodal, immunosuppressive regimen was started 1 day after the skin biopsy, before further histopathologic diagnosis.
Treatment
On the day of presentation to the referral clinic, acetaminophen (ie, paracetamol; 10-20 mg/kg PO every 12 hours) and gabapentin (10-20 mg/kg PO every 6-8 hours) were administered as needed. Once the patient was able to tolerate administration, topical 4% chlorhexidine gel and 4% chlorhexidine foam were applied to lesions every 12 hours. Cephalexin (20 mg/kg PO every 12 hours for 10 days) was prescribed, and a premium, fixed-formula, dermatology-based, complete diet was initiated.
Three days after presentation to the referral clinic, pulse therapy was initiated with prednisolone at a very high dose (10 mg/kg PO every 24 hours for 3 days, then 2 mg/kg PO every 24 hours thereafter).3 Mycophenolate mofetil (15 mg/kg PO every 12 hours) and colchicine (0.015 mg/kg PO every 24 hours for 3-5 days, then 0.03 mg/kg PO every 12 hours thereafter) were administered. Omeprazole (1.2 mg/kg PO every 12 hours for 15 days) was prescribed to be administered 6 hours after mycophenolate mofetil. Oral sucralfate can be administered every 8 hours within 1 hour of feeding. Maropitant (≈1 mg/kg PO every 24 hours) can be administered as necessary.6
A combination of high-dose glucocorticoids and mycophenolate mofetil is the mainstay for suppressing the immune-mediated attack on the basement membrane. The initial very-high prednisolone dose is a well-established induction protocol for pemphigus foliaceus.3 Colchicine was added to reduce neutrophilic inflammation.2 Initial pain relief plus treatment for secondary microbial infection, as well as prevention of further secondary microbial infection, were considered paramount additional measures. Gastroprotectants and antiemetics were also used to reduce iatrogenic adverse effects.
Treatment at a Glance
Analgesia
Acetaminophen (ie, paracetamol)
Gabapentin
Antimicrobial
Topical (4% chlorhexidine gel and 4% chlorhexidine foam)
Systemic (cephalexin)
Nutrition
Premium-quality, fixed-formula, dermatology-based, complete diet
Immunosuppressive
Very-high-dose prednisolone
Mycophenolate mofetil
Colchicine
Gastroprotectant & antiemetic
Omeprazole
± sucralfate
Maropitant (as needed)
Outcome
Over the following 3 months, daily prednisolone was gradually reduced and eventually stopped. Mycophenolate mofetil, colchicine, and all systemic and topical treatments were also able to be discontinued. After 3 years, no recurrence was reported.

FIGURE 11 Concave pinna 16 weeks after treatment
Conclusion
Canine epidermolysis bullosa acquisita is an autoimmune blistering skin disease directed against collagen VII.7,8 Male dogs are more often affected than females, and there is a breed predisposition in Great Danes.1,2 Many cases respond well to immunosuppressive treatment. Although later recurrence is possible, long-term treatment is not typically required.