Image used with permission from JAVMA
Therapeutic joint injection (TJI) is a common interventional therapeutic procedure that concentrates medication at the source of disease, potentially mitigating pain and inflammation while reducing systemic adverse effects of certain drugs.1 Traditional TJIs include corticosteroids, hyaluronic acid products, and local anesthetics. Orthobiologics (eg, stem cells, platelet-rich plasma [PRP], bone marrow aspirate concentrate), various hydrogels, and some radioactive agents (via radiosynoviorthesis) have been explored for treatment of osteoarthritis (OA) in dogs and horses.2-5
What are the considerations for patient selection, indications, and contraindications?
TJI has been used for treatment of rheumatoid arthritis in humans and treatment of OA in humans and animals.6-11 OA is a prevalent, lifelong, progressive disease; confirmed diagnosis is the most common indication for consideration of TJI in veterinary patients.12-14 Systemic pain medications (eg, NSAIDs) can have adverse effects, and TJIs may reduce the use of potentially harmful systemic medications over time.12 TJIs may also provide an alternative to NSAIDs in patients with GI, liver, or renal disease and can provide adjunctive pain relief when traditional medical management alone is insufficient.
Safety and efficacy of any medical therapy or intervention should be discussed with the pet owner. TJI has been shown to be relatively safe when used judiciously with sound medical techniques.15 Adverse effects of TJI and specific injectates include iatrogenic tissue damage, transient pain, joint flare, and septic arthritis.9,15,16 TJI-related septic arthritis is not commonly reported in horses, dogs, or humans, but a recent study demonstrated that transient gastroenteritis (6.8%) and injection site joint soreness (18%) were relatively common in dogs.15 Joint flare is a subjective description of more marked, transient, acute-onset soreness that can occur with almost any injectate; however, stem cells have been reported to have a higher incidence of postprocedural discomfort compared with other injectates in dogs.15,17 Joint flare typically occurs within a few days after administration, is relatively transient, and usually responds well to pain management.9 Sterile joint inflammation may need to be differentiated from septic arthritis through clinical evaluation and other diagnostic measures. In general, TJI complication rate and severity may be increased in patients that are immunocompromised or experiencing certain pathologies (eg, cancer, infection) within the target joint.
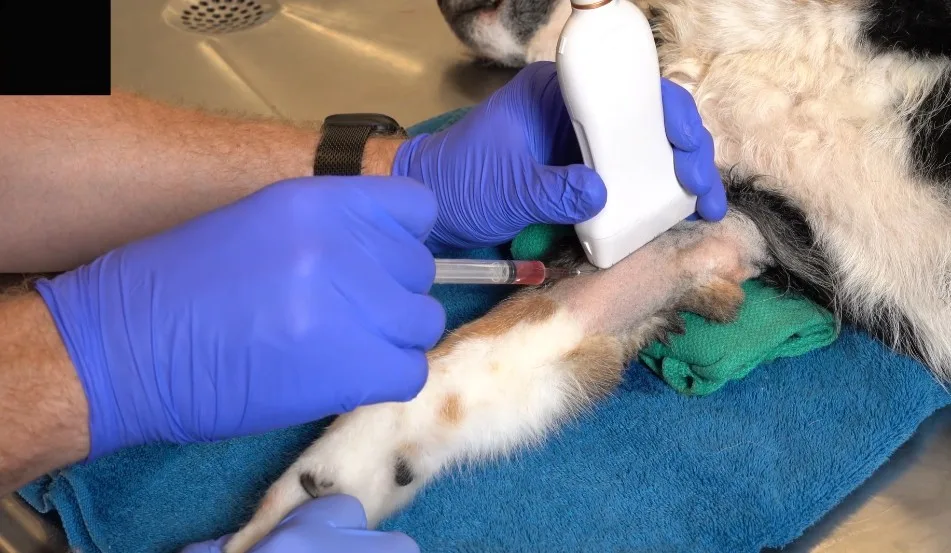
TJIs have historically been administered blindly via percutaneous palpation with standard antiseptic preparation over the clipped area.18 Aspiration of synovial fluid can help confirm needle placement within the joint prior to injection. In human medicine, ultrasonography to guide delivery of intra-articular treatment has been shown to reduce discomfort and iatrogenic damage and to improve accuracy and therapeutic outcomes.19-21 A study in dogs demonstrated iatrogenic damage to cartilage and a significant lack of accuracy with landmark-guided shoulder injections by orthopedic experts.16 Imaging guidance is therefore recommended when possible, particularly for deeper joints.22
What are some common TJIs and considerations regarding use?
Consideration for the use of TJI products is based on limited clinical and safety data in dogs, as well as data extrapolated from human medicine. Injectate should not be forced into a joint if resistance is felt during administration.
Corticosteroids
Corticosteroids are commonly administered intra-articularly to reduce synovitis and related joint pain.
Intra-articular insoluble corticosteroids have been shown to provide clinical benefit ranging from 1 to 12 weeks in horses and humans.6,23-25
In dogs, typical intra-articular doses of triamcinolone acetonide range from 0.2 to 0.25 mg/kg total for 1 joint or divided between 2 joints.
Some systemic absorption occurs with all intra-articular corticosteroids, but these effects were well tolerated in healthy dogs given triamcinolone acetonide at 0.25 to 0.5 mg/kg.26
In humans, it is not recommended to administer >3 corticosteroid injections per year in a single joint because both local (eg, tissue atrophy, cartilage damage) and systemic (eg, hyperadrenocorticism, diabetes, immunosuppression) complications can occur.6,26
The author administers intra-articular triamcinolone acetonide up to once per year for synovitis flares (acute-on-chronic worsening of the OA process) in patients with OA.
Intra-articular corticosteroids should be avoided or used with caution in dogs with a history of gastroenteritis or recent NSAID administration.
Discontinuing NSAIDs within 5 days of administration and/or providing omeprazole for ≈10 days after intra-articular triamcinolone acetonide administration may reduce the risk for gastroenteritis, but this has not been studied.
Hyaluronic Acid
Hyaluronic acid is a nonsulfated glycosaminoglycan, a normal component of articular cartilage and synovial fluid, and considered a viscosupplement with anti-inflammatory properties.27
Many studies support higher molecular weight hyaluronic acid (3,000-6,000 kDa) rather than lower molecular weight products for treatment of clinical OA; the duration of effect can last up to 1 year.28-32
In a small, controlled study involving a surgically induced model of OA in dogs, both formulations of hyaluronic acid (500-750 kDa and 6,000 kDa) improved weight-bearing on the affected limb compared with placebo. The higher molecular weight product showed greater benefit,33 suggesting a lower molecular weight product may still provide clinical benefit.
Hyaluronic acid is often used in combination therapy (with concurrent PRP or corticosteroids) despite limited evidence of added benefit; however, excessive joint volumes should be avoided, particularly in smaller joints, as this may cause discomfort or lameness.15
Platelet-Rich Plasma
Autologous PRP is an orthobiologic produced by enriching platelet concentrations within plasma by processing whole blood, most often by centrifugation.34
PRP is one of the TJIs most studied in dogs, and multiple studies have demonstrated its efficacy and acceptable safety for intra-articular use in patients with OA.3,35-38
Autologous PRP is simple to produce, deliverable on-site, and relatively affordable. Platelets are rich with growth factors that are released upon activation with the goal of promoting healing and mediating inflammation at the target.
Optimal product composition remains unknown. There is marked variability between and within products. Gaps exist within the current literature; however, leukocyte-reduced PRP may be more ideal for joints.34,39-42
Intra-articular PRP may provide clinical relief for ≈3 months.3
Limitations in Knowledge
The true risks for iatrogenic damage and long-term sequelae from intra-articular TJI and traditional injectates are unknown.
There is a paucity of data available for dogs; most studies focus on humans and horses.
Risk may depend on technique, clinician experience, equipment, and joint size, among other variables.
Needle damage to articular cartilage may be more concerning in younger patients with a less affected joint than in older patients with end-stage OA.
Ultrasound-guided TJI has demonstrated reduction of risks in humans but has not been explored in dogs.
Insoluble corticosteroids and hyaluronic acid have little to no controlled efficacy trials in dogs, and much of the dosage data are based on limited canine clinical and safety studies, as well as data extrapolated from human medicine.6,26,33,36,43
Although intra-articular PRP has been studied more often than these other injectates in dogs, variability in dosages, target site, research bias, and study population make definitive clinical recommendations challenging.3,42
For any intra-articular injectate, some systemic absorption is likely, particularly in diseased joints.44,45
Intra-articular corticosteroids (eg, insoluble triamcinolone) can be detected in the synovial fluid of dogs and horses for up to 6 weeks at varying doses.46-49
Intra-articular insoluble triamcinolone acetonide at clinical doses may have mild and transient systemic effects for ≈1 week in dogs and is not detectable in plasma after ≈1 week in horses,26 which differs from longer durations of plasma detection when triamcinolone acetonide is administered intramuscularly.46-49
The amount and duration of medication within the local tissues are unknown and challenging to study.
Based on available literature, systemic administration of corticosteroids may have a therapeutic effect on diseased joints but may also have the potential for adverse systemic effects for a longer period compared with intra-articular administration due to the underlying pharmacokinetics.6,48-52
Whether the observed durations of therapeutic effects are primarily due to localized duration of medication or to an immediate effect of the injectate with lasting sequelae is unknown.
Video tutorials on ultrasound-guided arthrocentesis and intra-articular injections of the elbow and stifle joints and of the hips and shoulder joints in dogs are available (see Suggested Reading).
Conclusion
TJI may be a promising treatment option in some patients, but recognizing limitations in current scientific research, particularly in dogs, is important. The potential risks and benefits should be considered for each patient and conveyed to the owner.
More research is warranted, and recommendations for TJI indications, contraindications, dosages, and administration techniques may change over time. Incorporating TJI into the typical skill set should include continuing education, further reading of evidence-based studies, and participation in wet labs led by experts.