Respiratory Support for Acute Intensive Care
Lisa L. Powell, DVM, DACVECC, BluePearl Veterinary Partners, Eden Prairie, Minnesota
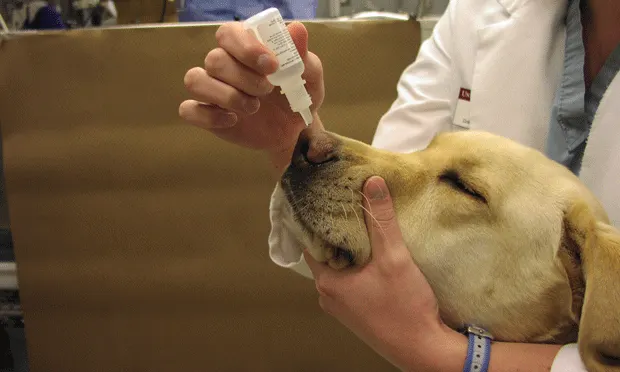
Respiratory disease is a frequent cause of illness in small animal veterinary patients. In such cases, oxygen administration is always indicated as a means of acute clinical support of the pulmonary system. Ideally, the method used to administer oxygen should induce minimal stress while increasing the fractional inspired oxygen concentration (FiO2) in the patient's environment.
The inspired concentration of oxygen in ambient air is 0.21, or 21% (FiO2 = 21%). Depending on the method of oxygen administration and flow rates used, the FiO2 in the patient's inhaled environment can range from 30% to 100% (Table). Methods commonly used include flow-by or mask O2, an enclosed oxygen cage or neonatal incubator, and unilateral or bilateral nasal cannulas.

There are 5 general causes of hypoxemia: hypoventilation, ventilation/perfusion (V/Q) mismatch, intrapulmonary shunt, diffusion impairment, and decreased inspired oxygen concentration. Low FiO2 concentrations are not usually a pathologic cause of hypoxemia. For example, FiO2 levels decrease at higher altitudes causing a relative hypoxemic state, until normal homestatic mechanisms cause adaptation to the new FiO2 levels.
Hypoventilation results in hypercapnia because of the patient's inability to expire carbon dioxide. Diseases associated with hypoventilation primarily include nonparenchymal lung diseases, such as neuromuscular diseases; cervical spinal cord disease; obstruction or disease of the major airways; general anesthesia or heavy sedation; and severe respiratory muscle fatigue or weakness. Because the pulmonary tissue is normal in cases of pure hypoventilation, gas exchange at the alveolar-capillary interface is unaffected, and administration of oxygen relieves the hypoxemia. However, the patient remains hypercapnic unless artificial ventilation is administered, or the underlying disease is addressed.
V/Q mismatch is a common cause of hypoxemia in small animal veterinary patients. It occurs when ventilation and blood flow in the lungs are unequal, resulting in inefficient transfer of oxygen from the alveoli to the pulmonary circulation and subsequent hypoxemia. Diseases that cause V/Q mismatch include pulmonary edema, pulmonary hemorrhage, pneumonia, pulmonary neoplasia, and pulmonary thromboembolism. Enriching the FiO2 in these patients increases the concentration of oxygen in the pulmonary alveolus, creating a larger diffusion gradient and improving blood oxygen levels.
Intrapulmonary shunt occurs when there is severe consolidation or atelectasis of the lung, resulting in hypoxemia that is less responsive to oxygen administration: The lung involved is not being ventilated at all because of collapse or severe disease, so enriching the oxygen environment does not improve blood oxygen levels. However, oxygen administration is still indicated in these cases.
Diffusion impairment occurs when the alveolus-blood barrier is thickened because of disease, such that oxygen cannot equilibrate as red blood cells traverse the pulmonary vasculature. Interstitial diseases, such as pulmonary fibrosis, cause hypoxemia due to diffusion abnormalities. Diffusion impairment is a less common cause of hypoxemia in small animal patients.
Assessment of Oxygenation
Objective measurement of ventilation and oxygenation is best obtained by evaluation of arterial blood gas measurements. However, pulse oximetry is much more readily available than blood gas analyzers and is therefore probably used more frequently in small animal practice. Pulse oximetry measures the percentage of hemoglobin that is saturated with oxygen, an indirect measure of arterial oxygen levels. As the pulse oximeter readings decrease below 91%, oxygen levels drop sharply, according to the oxyhemoglobin saturation curve (Figure 1).

Oxyhemoglobin saturation curve. P50 = 50th percentile; PO2 = partial pressure of oxygen
Pulse oximeters do have limitations, including erroneous readings with peripheral vasoconstriction/low tissue perfusion, pigmentation of mucous membranes, and increased ambient lighting. When a pulse oximeter reading is assessed, the heart rate measured by the machine should match the patient's true heart rate, and a good waveform and strong signal should be verified.
In addition, hemoglobin abnormality (ie, carboxyhemoglobin and methemoglobin) will result in inaccurate pulse oximetry readings and should not be assessed in these situations. Carboxyhemoglobin is most often due to smoke inhalation injury, resulting in elevated levels of carbon monoxide in the blood. Methemoglobin most often results from acetaminophen toxicity, especially seen in cats. Hemoglobin is oxidized from its ferrous state (Fe2+) to ferric (Fe3+) iron, which is incapable of carrying oxygen. Another drawback to the use of the pulse oximeter is the inability to assess blood carbon dioxide levels; thus, ventilation cannot be evaluated.
Finally, anemia may result in a pulse oximetry reading that is higher than the actual oxygenation status of the patient because of relatively fewer oxygen-binding sites available. Polycythemia might lead to an opposite reaction because there would be an abnormally increased number of oxygen-binding sites available for a normal number of oxygen molecules in a healthy patient.
Oxygen Therapy
As previously stated, when oxygen is administered to a patient in respiratory distress, the method used should increase the level of FiO2 while inducing the lowest amount of stress to the patient.
Mask or flow-by O2 provides for a very high FiO2, but unless the patient is recumbent with decreased mentation, it is not a functional method for administering oxygen on a long-term basis. This method is primarily used for patients that present in respiratory distress and require immediate oxygen therapy while being assessed for the cause of the pulmonary dysfunction. Mask or flow-by O2 can also be used during procedures such as thoracic radiography, thoracocentesis, and transtracheal wash. When flow-by O2 is being administered, the oxygen flow should not be directed into the patient's face because many animals dislike the sensation. In addition, oxygen masks can induce undue stress and should not be used if the patient begins to resist. Flow-by O2 can be administered horizontally, in front of the animal's nose; this is usually tolerated very well in dyspneic patients.
An enclosed oxygen cage or neonatal incubator is the preferred method for oxygen administration in cats with respiratory distress. An FiO2 of approximately 40% can be achieved by using this method. Many commercial oxygen cages provide the FiO2 value in the cage environment and also regulate humidity and temperature in the cage. The disadvantages of incubators or unregulated oxygen cages include an unknown FiO2 concentration, no humidification of the oxygen administered (unless the oxygen is run through a water source), and the risk for overheating due to the enclosed environment of the cage/incubator. For these reasons, a commercial oxygen unit is recommended. When an oxygen cage or incubator is used for patient oxygen support, treatments should be combined so that the number of times the doors are opened is minimized.
Nasal oxygen administration through the use of unilateral or bilateral nasal cannulas is the recommended form of oxygen administration for dogs. Once they have been placed, nasal cannulas provide a consistent, high level of oxygen flow with minimal stress to the patient. In patients with extreme respiratory distress, nasal cannulation may be too stressful a procedure, so placement in an oxygen cage is preferred. With nasal cannulation, flow rates usually cannot exceed 2 L/min with one cannula and 4 L/min with bilateral cannulas, allowing for FiO2 levels of approximately 40% to 60%, depending on the number of cannulas and oxygen flow rates. Higher flow rates cause discomfort of the nasal mucosa from the forceful jet of oxygen through the tubing. Disadvantages of oxygen administration through nasal cannulas include the stress involved with placement, the tendency for easy removal through sneezing or pawing at the area, and clogging of the tube with nasal secretions after a prolonged period of use.
Step-by-Step: How to Place Nasal Cannulas
What You Will Need
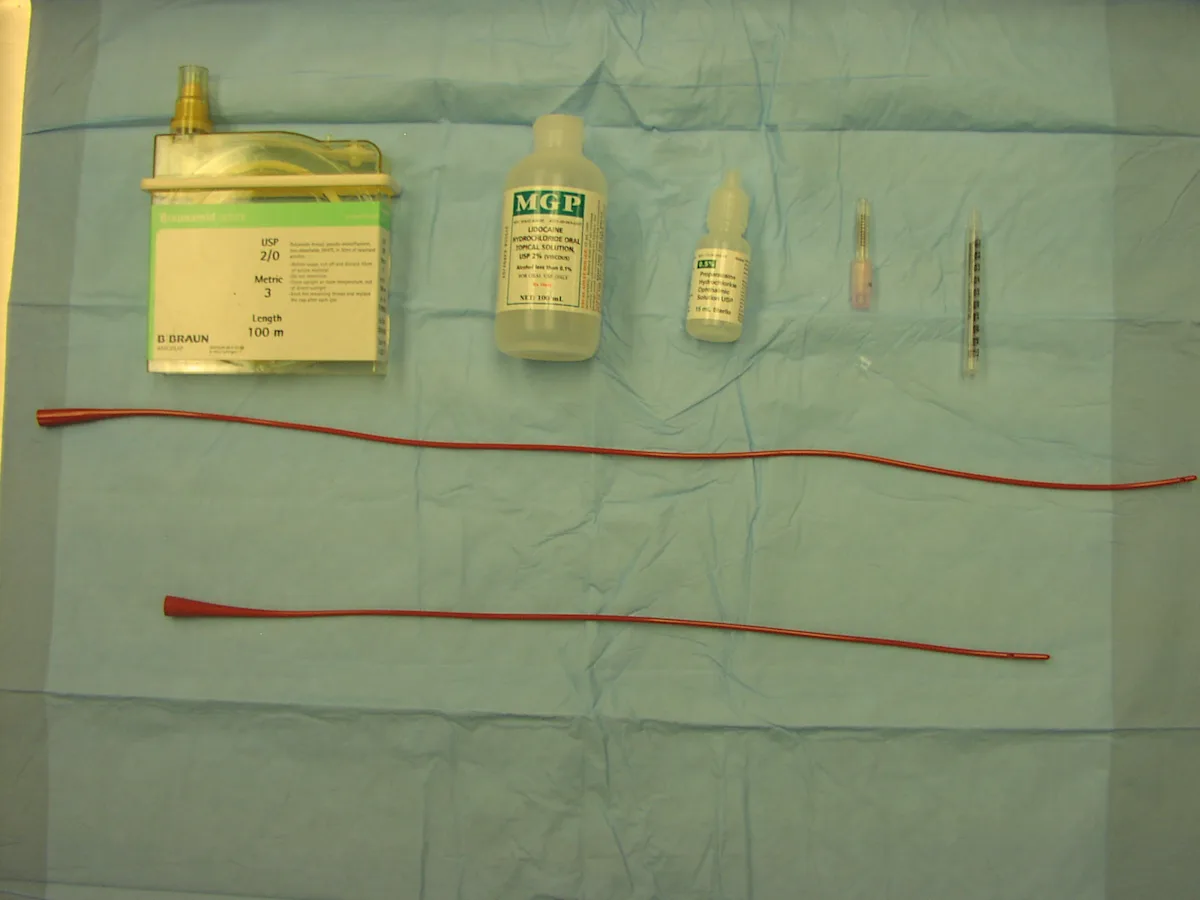
Nonabsorbable suture* 20-gauge needle
Ocular proparacaine
Lidocaine gel
1-ml syringe with the base of the syringe cut off
8-French (or 5-French for smaller dogs) red rubber tube (56 cm long for larger dogs, 41 cm for smaller dogs)
Step 1
Place 2 to 3 drops of ocular proparacaine into the nose.
Procedure Pearl
Flow rates usually cannot exceed 2 L/min with one cannula and 4 L/min with bilateral cannulas.

Step 2
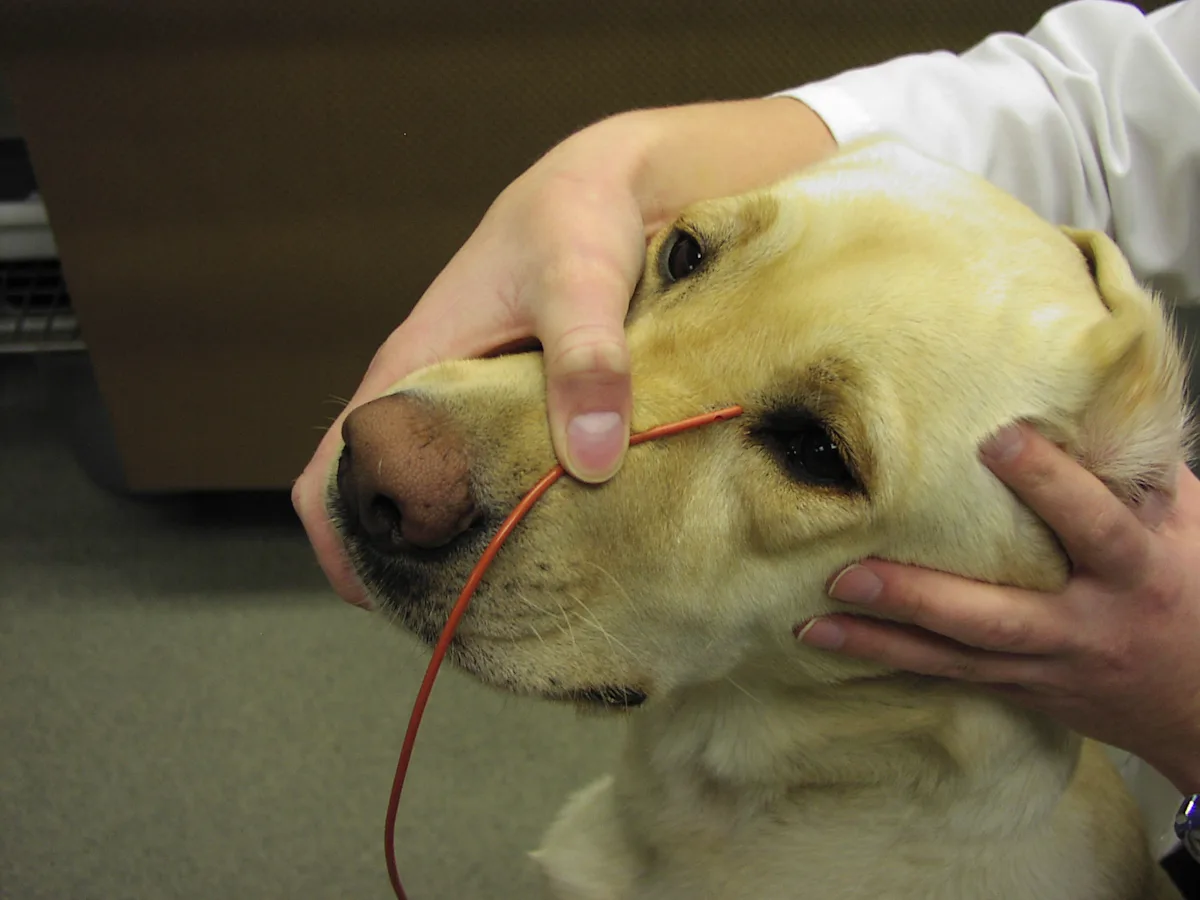
Measure red rubber tube to the medial canthus of the eye.
Step 3
Mark the tube at the point measured to the medial canthus of the eye.
Procedure Pearl
Nasal cannulas are the recommended form of oxygen administration for dogs.

Step 4
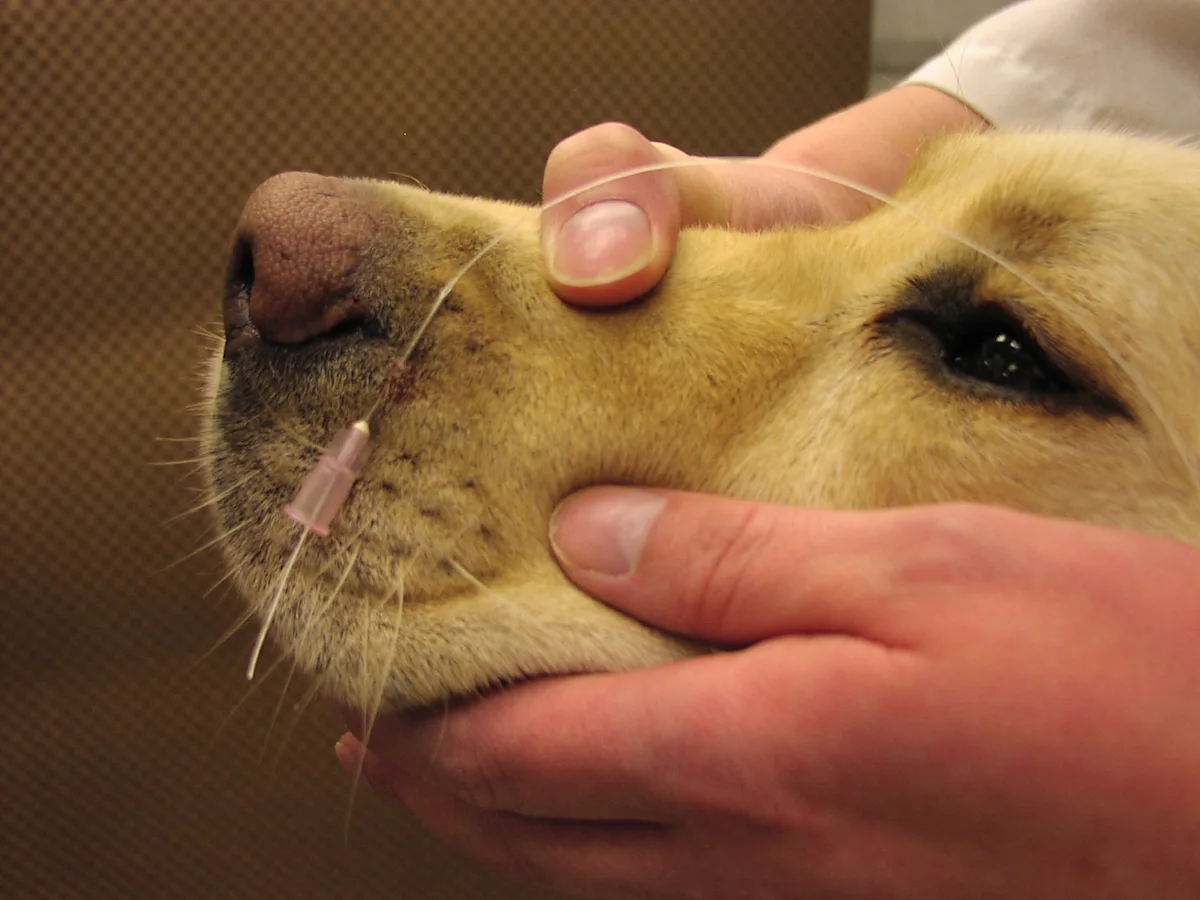
Place a 20-gauge needle through the skin immediately lateral to the nares being used to place the tube. Thread nonabsorbable suture through the needle, pulling the suture through the sharp end of the needle first. Once it is threaded, pull out the needle and place a double knot onto the skin, leaving both ends of the suture long.
Step 5
Repeat the procedure described in step 4, placing a skin suture with long ends at the base of the ear.
Procedure Pearl
Leaving both ends of the suture long, place a double knot onto the skin.

Step 6
Apply lidocaine gel to the end of the red rubber tube, and place the tube into the nasal canal, aiming ventrally, to the measured mark on the tube. Suture the tube next to the nose by using a finger-trap suture pattern. Then suture the tube at the second site, the base of the ear, using a single knot or finger-trap pattern. Alternatively, the tube can be sutured to the top of the head, although this sometimes causes the tube to fall in front of the eyes, disturbing the patient.
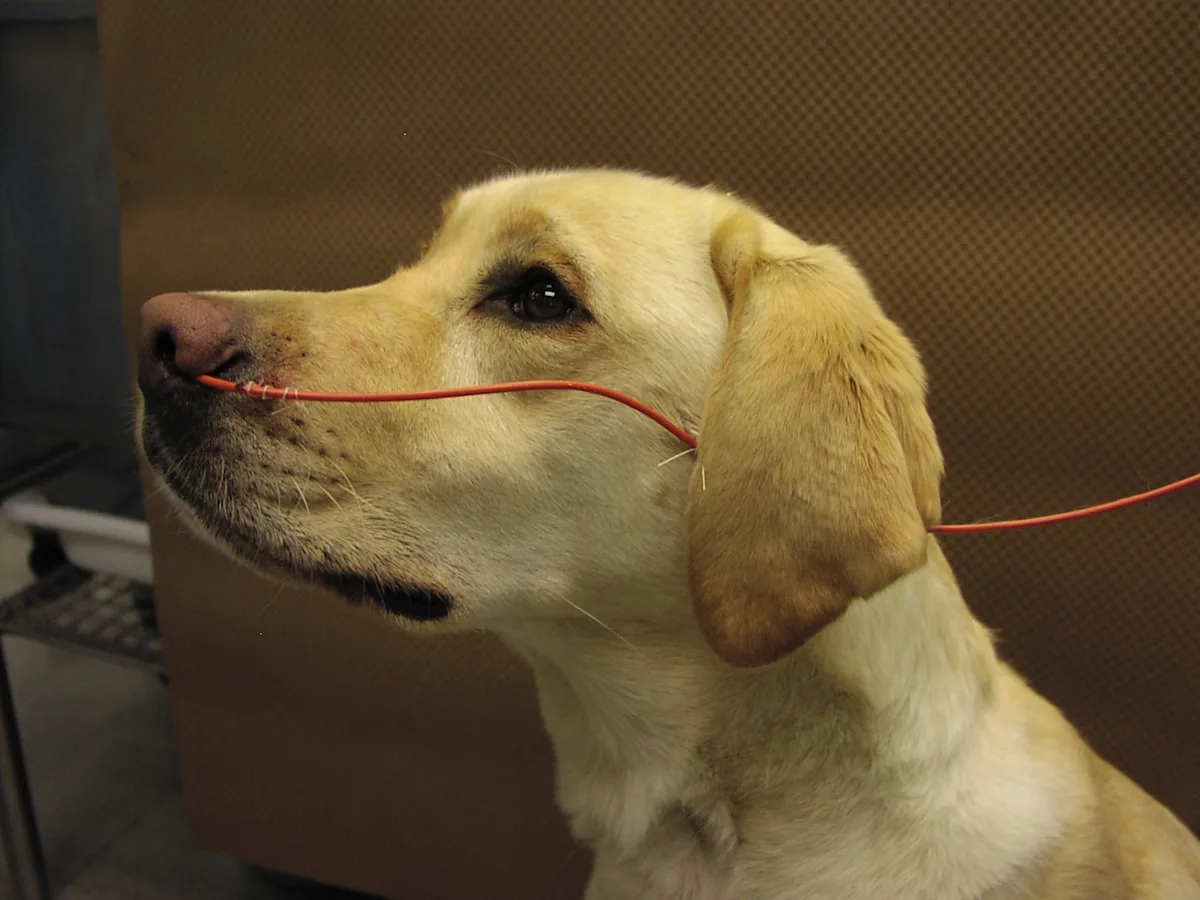
Step 7
Always place an Elizabethan collar on dogs with nasal cannulas because the cannulas are easily removed by pawing at the area.
Procedure Pearl
Always place an Elizabethan collar on dogs with nasal cannulas.

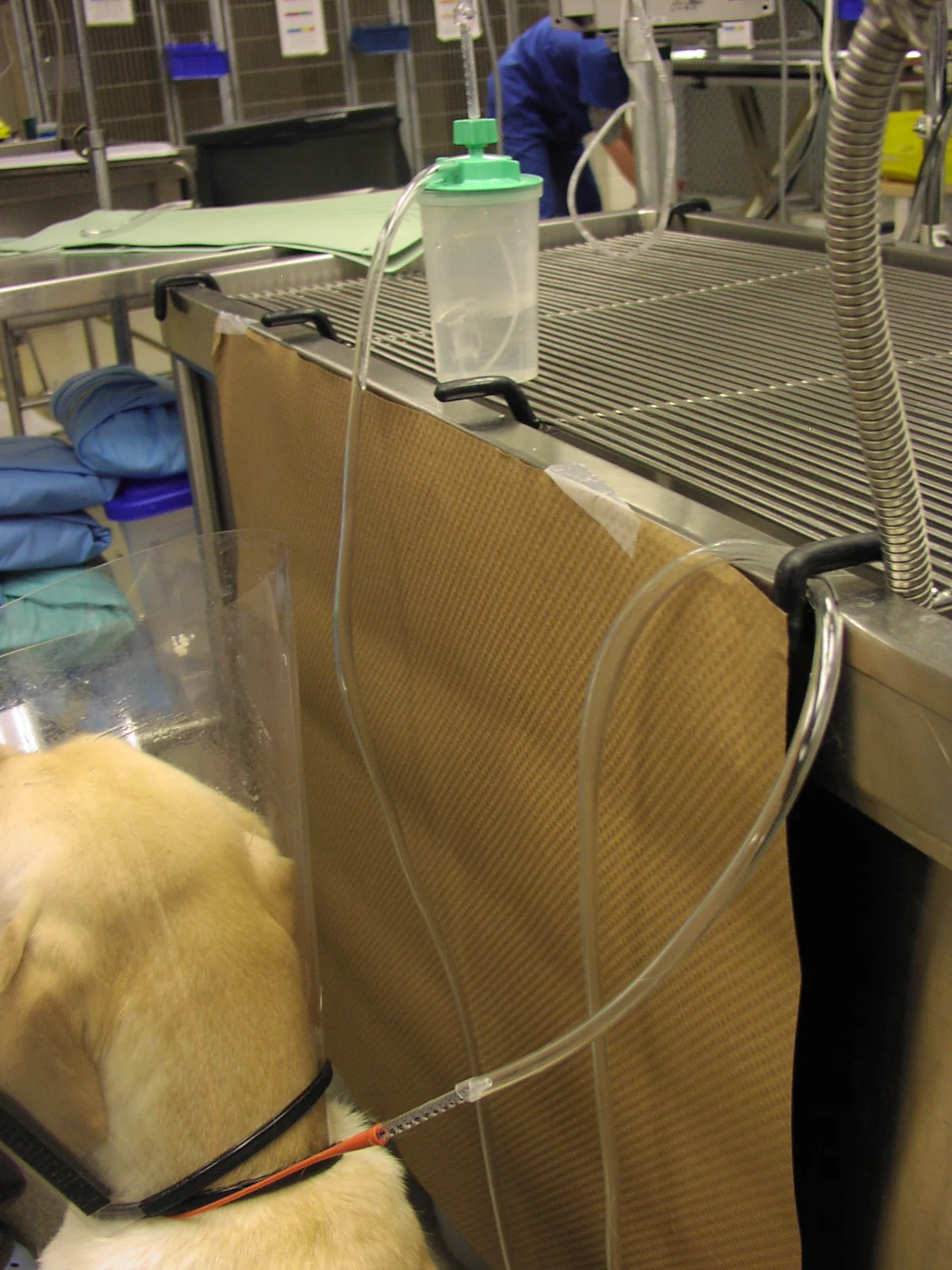
Step 8
Attach the tube to the cut 1-ml syringe, attach it to the oxygen tubing, then to a water source to humidify the oxygen, and finally, to the source of oxygen.
Conclusion
Oxygen administration becomes imperative for the treatment of critically ill patients with pulmonary dysfunction. Methods of enriching a patient's oxygen environment should cause minimal stress while providing the highest percentage of oxygen in a consistent manner. Patients with significant respiratory distress and hypoxemia despite oxygen administration are candidates for sedation, intubation, and manual ventilation.