Proteinuria in Dogs and Cats
Profile
DefinitionProteinuria is excess protein in urine. It may be prerenal, renal, or postrenal in origin.
Prerenal proteinuria results when small proteins that are not normally free in the plasma or abnormal proteins pass through the filtration barrier into the urine.
Renal proteinuria arises from within the kidneys. It may be functional (physiologic) or pathologic.
Functional proteinuria is mild and transient, resolving when the inciting causes are eliminated (see Causes). Functional protein is poorly documented in dogs and cats.
Pathologic proteinuria is a consequence of structural or functional renal lesions and may be classified as glomerular, tubular, or interstitial.
Glomerular: Results from glomerular capillary lesions altering the permselectivity of the glomerular barrier.
Tubular: Results from impaired ability of the proximal renal tubules to reabsorb proteins normally passing the glomerular barrier.
Interstitial: Results from exudation of proteins into urine due to renal interstitial inflammation or disease.
Postrenal proteinuria occurs when protein infiltrates urine as a result of hemorrhage or inflammation in the lower urinary tract. It may also be added from the genital tract or genitalia after urine has left the urinary tract, in which case it is termed extraurinary proteinuria. Nephrotic syndrome is characterized by severe glomerular proteinuria, hypoalbuminemia, hypercholesterolemia, and edema (Figure 1).

Figure 1. Hindlimb edema characteristic of nephrotic syndrome
Systems. Pathologic proteinuria is evidence of urinary tract disease. However, proteinuria often occurs consequent to diseases in other organ systems, including extrarenal chronic inflammatory, infectious, or neoplastic processes or altered renal hemodynamics.
Genetic Implications. Proteinuria may occur as a sign of congenital glomerulopathies (in cocker spaniels, bull terriers, dalmatians, Doberman pinschers, beagles, rottweilers, Pembroke Welsh corgis, Newfoundlands, and bull mastiffs), hereditary immune-mediated glomerulonephritis (soft-coated wheaten terriers, Bernese mountain dogs, and Brittany spaniels), familial reactive systemic amyloidosis (shar-peis, beagles, English foxhounds, and Abyssinian cats), and renal dysplasia (shih tzus, Lhasa apsos, golden retrievers, standard poodles, miniature schnauzers, Alaskan malamutes, soft-coated wheaten terriers, chow chows, and Dutch Kooikers).
Incidence/Prevalence. Incidence and prevalence poorly documented. Prevalence of microalbuminuria in clinically healthy dogs and cats estimated at 19% to 25% and about 15%, respectively.
Causes
Prerenal proteinuria is most often due to hemoglobinuria, myoglobinuria, or Bence Jones proteinuria.
Functional renal proteinuria occurs with pyrexia, extremes of heat and cold, strenuous exertion, seizure, and stress.
Pathologic glomerular renal proteinuria results from glomerular lesions impairing glomerular filtration of proteins.
Pathologic tubular renal proteinuria results from tubular diseases that impair tubular uptake of small proteins. Pathologic interstitial renal proteinuria results from infectious or noninfectious inflammatory diseases of the renal interstitium.
Postrenal proteinuria occurs due to urinary tract infections (occurring anywhere in the urinary tract including the kidneys), uroliths, urinary neoplasia, or urinary trauma.
Risk Factors
Chronic inflammatory, infectious, or neoplastic diseases
Systemic hypertension
Hyperadrenocorticism
Chronic kidney disease
Acute kidney injury
Hematuria and pyuria
Myopathies
Multiple myeloma
Pyrexia
Extremes of heat or cold
Epilepsy
PathophysiologyThe glomerulus is a high-pressure capillary tuft that allows production of a nearly protein-free filtrate. Proteins cross to the tubular fluid in inverse proportion to their size and negative charge. Proteins with a molecular weight of less than 20,000 Daltons pass easily across the glomerular capillary wall. Conversely, albumin, with a molecular weight of approximately 65,000 Daltons and a negative charge, is restricted.
Smaller proteins may pass through the glomerulus but are normally reabsorbed by the renal tubules before excretion. Tubular proteinuria occurs when tubulointerstitial disease prevents the proximal tubule from reabsorbing low-molecular-weight proteins normally present in the glomerular ultrafiltrate.
Glomerular capillary walls -- consisting of endothelial cells, the glomerular basement membrane, and visceral epithelial cells -- form a barrier to passage of some plasma proteins. In glomerular proteinuria, disturbances of the permselectivity function of this barrier in regard to size or electrical charge of molecules lead to leakage of albumin and larger plasma proteins into the glomerular ultrafiltrate. Immune mechanisms are central to the pathogenesis of most types of glomerulonephritis. In such cases, immunoglobulins and complement factors can be demonstrated on glomerular structures. Antibody-mediated glomerular injury may result from deposition of circulating immune complexes in the glomerular capillary wall or binding of antibodies directed against antigens present in the glomerular wall (in situ immune complexes).
Diagnosis
Diagnosis of proteinuria includes differentiating between prerenal; postrenal; and functional, pathologic glomerular, pathologic tubular, and pathologic interstitial renal proteinuria. Establishing the diagnosis requires detecting (screening), localizing, and quantifying the magnitude of proteinuria.
Screening
Screening tests for proteinuria cannot be interpreted without complete urinalysis results.
Preliminary diagnosis of proteinuria is usually based on urine dipstick results. This test is most sensitive to albumin and has a lower detection limit of 30 mg/dl. False-positive results can occur with alkaline urine, active urine sediment (pyuria, hematuria), quaternary ammonium compound contamination, or prolonged contact of dipstick with urine. False-negative results occur with Bence Jones proteins and dilute or acidic urine.
Confirm positive dipstick results by the SSA turbidity test. This test reliably detects albumin, globulins, and Bence Jones proteins. Presence of radiocontrast agents, penicillin, cephalosporin, sulfisoxazole, or thymol (a urine preservative) may cause false-positive SSA results. False-negative results may occur with highly buffered alkaline urine or a dilute specimen.
Proteinuria screening or confirmation of dipstick results may also be performed using the early renal damage test (E.R.D.-HealthScreen, www.heska.com) for microalbuminuria. Negative tests probably reflect true nonproteinuria, weak or moderately positive results indicate microalbuminuria, and strongly positive results suggest overt proteinuria.
Localizing
Rule out prerenal and postrenal proteinuria using history, physical examination, complete urinalysis and culture, and blood tests. Sample urine by cystocentesis to rule out extraurinary proteinuria.
Inflammatory urine sediment with concurrent clinical signs of active nephritis, such as pyrexia, painful kidneys, or kidney failure, is consistent with (but not conclusive proof of) pathologic interstitial renal proteinuria. However, many dogs with chronic pyelonephritis do not have any specific signs suggesting upper tract involvement. In such instances, recognition and confirmation of pathologic interstitial renal proteinuria requires additional diagnostic evaluations such as pyelocentesis with culture and cytology and intravenous pyelography or renal ultrasound.
Persistence of proteinuria with inactive urine sediment should be demonstrated on 3 or more occasions, 2 or more weeks apart. Proteinuria that is transient (ie, not persistent) and mild is most consistent with functional proteinuria. Persistent proteinuria indicates pathologic renal proteinuria.
Quantifying
Measure UPC ratio if inactive urine sediment and persistent proteinuria are present.
Interpretation of UPC ratios is summarized in the Table.
Follow-Up Diagnostics for Pathologic Renal Proteinuria
Monitor proteinuria regularly to detect changes in magnitude that may indicate the need to initiate or alter therapy.
Evaluate for chronic infectious, inflammatory, or neoplastic diseases and drugs/toxins that may be contributory. Identification and elimination of causative/associated antigens is a rational therapeutic goal.
Treatment
Inpatient or OutpatientManage on outpatient basis unless complicated by uremia or nephrotic syndrome
Medical Medical treatment includes ACE inhibitors (see Medications) and diet therapy.
Indicated for dogs and cats with azotemic chronic kidney disease (IRIS stages 2, 3 and 4) and UPC ratios greater than 0.5 or 0.4, respectively, and nonazotemic dogs and cats (IRIS stage 1) with UPC ratio values greater than
Specific therapy directed toward underlying causes.
Due to inadequate studies on cortico- steroids or immunosuppressive drugs, there are no specific recommendations for or against their use.
Nutrition Appropriate diets contain reduced high-quality protein, omega-3 fatty acids, and limited salt. Manufactured renal diets for dogs and cats are appropriate. Renal diets have been shown to be beneficial in dogs and cats with serum creatinine values greater than 2 mg/dl; evidence supporting a recommendation for diet therapy in proteinuric patients with creatinine values below 2 mg/dl is weak.
Client Education
Proteinuric renal diseases are often progressive and may result in substantial morbidity and mortality. Monitoring and therapy may markedly improve quality and quantity of life.
Instruct clients to look for and report signs of decreased appetite, weight loss, vomiting, behavioral or neurologic signs, sudden loss of vision, or edema.
Medications
Drugs
An ACE inhibitor (eg, enalapril, benazepril) is indicated to reduce proteinuria. The starting dosage for enalapril is 0.5 mg/kg Q 24 H; for benazepril it is 0.25 to 0.5 mg/kg Q 24 H. Dosage may be increased to 0.5 mg/kg Q 12 hours.
Measure serum creatinine concentration before beginning therapy and again 5 to 7 days after initiating therapy.
If serum creatinine increases more than 0.2 mg/dl, consider monitoring serum creatinine concentration again in 5 to 7 days. Consider reducing dosage or discontinuing ACE inhibitor therapy should serum creatinine concentration continue to increase or exceed an overall increase of 0.5 mg/dl.
Goal is to reduce UPC ratios below 1 or 50% less than baseline.
Amlodipine may reduce proteinuria in cats with systemic hypertension and proteinuria. Dose is 0.625 mg Q 24 H for patients up to 5 kg and 1.24 mg Q 24 H for those over 5 kg.
Contraindications/Precautions
ACE inhibitors contraindicated in dehydrated patients; use cautiously with concurrent congestive heart failure and renal disease.
Always monitor renal response to ACE inhibitors.
Complications of ACE inhibitors include gastrointestinal signs, hypotension, hyperkalemia, and impaired renal function.
Follow-Up
Patient Monitoring
Ideally, proteinuria should be monitored every 4 to 6 weeks until the therapeutic end point is reached and every other month for 6 months thereafter. If a satisfactory response to therapy is achieved and a stable, persistent reduction in proteinuria is demonstrated over the subsequent 6 months, proteinuria should be monitored at least every 3 to 4 months.
Monitor blood pressure as indicated. Since hypertension is likely to be particularly harmful to the kidneys of patients with proteinuria, it is desirable to measure blood pressure whenever the magnitude of proteinuria is measured. Patients with stable, minimal risk blood pressure values should have blood pressure measured at least every 4 months.
Complications Complications include progressive renal dysfunction and kidney failure and nephrotic syndrome with edema, thrombotic diathesis, and hypertension.
Course
Most patients with severe proteinuria have progressive disease leading to renal failure or nephrotic syndrome.
In patients with mild to moderate proteinuria, prognosis for progression is more variable.
Proteinuria promotes progression of chronic kidney disease, shortening survival times.
In General
Relative Cost
Yearly monitoring costs for proteinuria that does not require medical management: $$
Yearly treatment and monitoring costs for pathologic glomerular renal proteinuria: $$$
Diagnostic workup for pathologic glomerular renal proteinuria: $$$$ Diagnostic workup and treatment for pathologic glomerular renal proteinuria and associated clinical signs: $$$$$
Prognosis
Mild to moderate renal proteinuria: Guarded long-term prognosis without therapy. Although empirical ACE inhibitor therapy appears to prolong survival, long-term survival with therapy remains guarded. Short-term prognosis typically guarded to good.
Severe glomerular proteinuria: Guarded to poor long-term prognosis without therapy. Therapy may improve quality of life and survival.

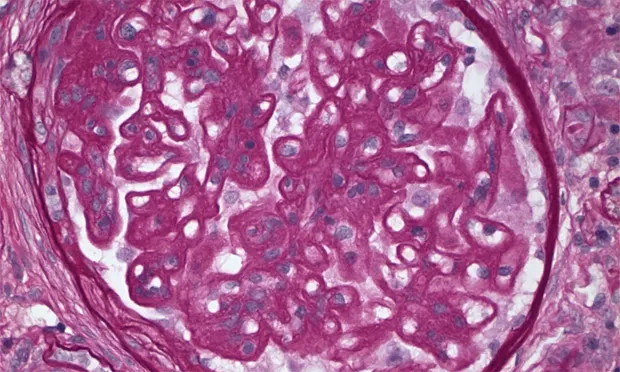
Future Considerations Studies on pathologic classification of proteinuric renal diseases in dogs are ongoing under the auspices of the World Small Animal Veterinary Association. Study goal is to identify clinical and pathologic manifestations of canine glomerulopathies (Figure 2). Prospective clinical trials to determine the effectiveness of therapies will follow.
Figure 2. Membranous glomerulopathy in an 8-year-old dog with proteinuric renal disease