Pain Management & Periodontal Disease
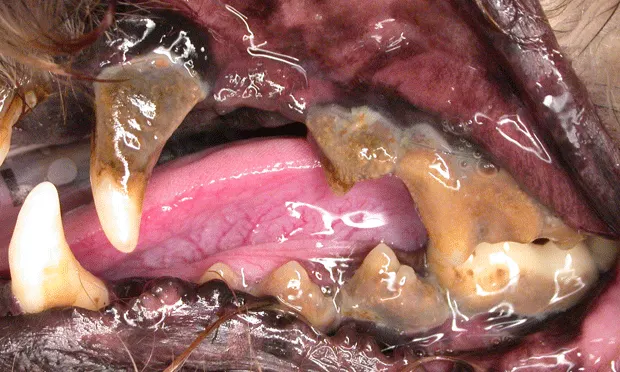
Figure 1 (above): Stage 4 periodontal disease. Local disease is obvious, with profound tartar accumulation, gingival recession, and purulent debris and plaque present in the sulcus.
ProfileDefinitionPeriodontal disease is plaque-induced inflammation of the supporting tissues of the teeth; it can destroy the gingiva, periodontal ligament, cementum, and alveolar bone. Associated pain results from tissue effects of inflammatory cytokines and chemokines produced by periodontal pathogens and the host's own immune system.
Systems. Generally considered a primary disease of the tissues around the teeth; however, systemic disease (eg, diabetes mellitus, Cushing's disease, FeLV, FIV, renal and hepatic insufficiency) can exacerbate primary disease, especially in states of immunocompromise.
SignalmentSpecies. Dogs and cats are both highly susceptible to periodontal disease. Cats may have exacerbated pain due to coexisting feline tooth resorption.
Breed Predilection. Small-breed dogs are particularly susceptible. Overcrowding and malocclusions play a role in progression in many of these patients. Greyhound, Maltese, and miniature schnauzer dogs tend to have more severe manifestations of rapidly progressive and refractory periodontitis. Somali and Abyssinian cats are overrepresented.
Age and Range. Severity and discomfort generally increase with age because of the progressive nature of the condition.Pathophysiology
Gram-negative anaerobic bacteria incite tissue inflammation, resulting in the release of adenosine triphosphate, potassium ions, hydrogen ions, prostaglandins, bradykinin, and nerve growth factors from damaged tissue.
Lymphocytes, monocytes, macrophages, and mast cells are attracted to the site, where they release cytokines, such as histamine, that enhance vasodilation and subsequently increase inflammation.
Allodynia results, whereby even touch or mastication may produce a pain response at the site of periodontitis.
Chronic inflammation may result in central sensitization and wind-up pain. In cases of chronic oral pain, neurons in the brain stem (nucleus caudalis) are chemically stimulated, enhancing the frequency and intensity of the pain signal to the brain. Glutamate and other chemicals bind to the NMDA receptor, sensitizing the postsynaptic neuron and adding to the local pain response. Specific NMDA-receptor antagonists, such as ketamine, can be used to counter this wind-up component of pain.
SignsHistory. Patients commonly exhibit no systemic clinical signs that are attributed to periodontal disease. Slowly progressive behavioral changes are often recognized only after the pain associated with periodontal disease resolves following proper surgical treatment.

Physical Examination. Local disease is often obvious; signs include gingivitis, calculus, gingival recession, pocket formation, mobile teeth, missing teeth, bleeding upon probing, purulent debris in the sulcus, and halitosis (Figures 1 and 2).
Figure 2: Radiograph of the left mandible of the patient in Figure 1.

Profound bone loss surrounds the roots of the premolars and molars. The patient's activity level dramatically improved after surgical correction with full mouth extractions.
Local tissue may appear normal despite underlying disease, defining the need for periodontal probing and radiography (Figures 3-5).

Figure 3: No obvious evidence of periodontal disease is present in this patient.
Figure 4: Radiograph of the right mandible of the patient in Figure 3. Bone loss is evident surrounding the roots of the fourth premolar. The patient was observably more active after surgery to correct the defect.

Figure 5: Surgical exposure of a defect in a patient similar to that in Figures 3 and 4. Granulation tissue is seen occupying the void created by periodontal bone loss (arrow).
Pain IndexPain from periodontitis is controversial. Most human dentists and periodontal literature attribute little pain to the disease except in the case of abscess or endodontic involvement. There is, however, little if anything published on this topic in the veterinary literature.
Severity of disease in humans is minimized by reasonable oral hygiene. In pets, accumulation of debris augments disease progression and many veterinary patients exhibit significant reflex response to even light periodontal probing under anesthesia beginning at stage 2 and heightening through the following stages.
No pain is present in stage 0 and 1 periodontal disease.
Discomfort is mild in some patients with stage 2 disease.
Moderate to severe pain is likely in stages 3 and 4.
DiagnosisDefinitive Diagnosis
Anesthesia is required for a complete definitive diagnosis of periodontal disease, including oral examination, periodontal probing, and dental radiography.
Periodontal pocket formation and bleeding are readily detected with a periodontal probe (Table).
Early radiographic changes include a decrease in density of interdental marginal bone, which in most cases is not painful.
Discomfort progresses as the periodontal ligament space widens or the height of marginal bone decreases, resulting in radiographically evident bone defects.
Differential Diagnosis
Canine or feline stomatitis
Feline tooth resorption
TreatmentInpatient or Outpatient
The success of periodontal treatment and elimination of the associated pain and discomfort depend on aggressive inpatient and outpatient management.
Preoperative and perioperative pain management, safe anesthetic maintenance, and definitive surgical correction are required for uneventful postoperative pain management and quick return to both comfortable mastication and periodontal home care.
Pain can be very difficult to assess in canine and feline patients; therefore, if doubt exists, treatment decisions should proceed as if pain were present.
Medical
Patients with chronic stage 3 and 4 disease should be expected to have a central pain component (wind-up). Failure to address this issue may complicate postoperative pain management.
Peripheral sensitization should also be expected and managed with analgesics, with special consideration given to NSAIDs.
Surgical
Surgical decisions are based on the owner's willingness and ability to perform periodontal home care and the commitment to return for scheduled periodontal therapy or prophylaxis in the hospital every 3 to 12 months.
Periodontal surgery to save a tooth in stage 3 or 4 disease should be attempted only if the pet owner is compliant.
Initial definitive treatment for stage 3 and 4 periodontal disease is surgical removal and contour of diseased bone and soft tissue.
Periodontal regeneration of tissues requires root surface biomodification, bone graft placement, or periodontal membrane utilization.
Extractions and gingival flap closure provide optimal healing after removal of diseased tissue.
Postsurgical pain varies because of individual tolerance, location of surgical site or sites within the oral cavity, number of sites, and preoperative or perioperative pain management (see Treatment at a Glance).
MedicationsDrugs/FluidsPreoperative and perioperative pain management includes the use of various drug classes to better manage postoperative pain:
Pure µ-receptor agonist opiates (eg, morphine, hydromorphone, and fentanyl) provide the most significant analgesia and should be used when moderate to severe pain is expected due to the severity of periodontal changes.
Buprenorphine, an opiate, is a partial µ-receptor agonist and provides a good alternative to the pure µ-agonists in anticipation of mild to moderate postoperative pain.
NSAIDs play an important role in the inflammatory component of pain and are a great adjunct to opiates.
Any of the preceding agents can be used in combination with low-dose medetomidine preemptively in otherwise healthy patients.
CRIs of ketamine and an opiate, following appropriate loading doses, can help to decrease the minimum alveolar concentration of the inhalant.
In addition, ketamine provides NMDA-receptor antagonism that helps counter central sensitization.
In dogs, lidocaine can be safely added to the CRI after an adequate loading dose.
Regional nerve blocks using lidocaine and bupivacaine with or without opioids are essential in oral surgery. Nerve blocks interrupt the transmission of noxious stimuli, thus reducing both peripheral and central sensitization and helping minimize the general anesthetic and postoperative analgesic requirements.
Precautions
NSAIDs should be used with caution in patients with concurrent gastrointestinal ulcerative diseases, renal or hepatic dysfunction, or hypoproteinemia. Blood pressure should be closely monitored in the intraoperative and immediate postoperative periods when NSAIDs are used.
Opiates should be used with caution in patients with hypothyroidism, severe renal insufficiency, and adrenocortical insufficiency.
Opiates, particularly hydromorphone, have been consistently shown to produce hyperthermia in cats. Therefore, temperature should be monitored for 5 hours after surgery.
Mu opiate receptor antagonists, such as nalbuphine, butorphanol, and naloxone, can be used to counter hyperthermia should it become significant.
Follow-upPatient Monitoring
Patient recovery should be monitored closely for signs of oral pain and the analgesic protocol for the individual adjusted as needed to provide maximal postoperative comfort.
Patients receiving regional nerve blocks are commonly pain free immediately after surgery and for several hours into recovery.
Additional analgesics will be needed after the activity of the local agent ceases.
Cats should continue to be monitored for hyperthermia during recovery.
At-Home Treatment and MonitoringAnalgesics are generally recommended for 4 days after surgery. Patients should be observed at home for signs of oral discomfort and medication regimens adjusted accordingly by the veterinarian. Pet owners commonly observe noticeable improvements in patient behavior after definitive care.
Mild Pain
An NSAID is probably all that is needed for mild pain in either species.
Moderate to Severe Pain
A pure µ-agonist opiate and an NSAID are considered effective for moderate to severe pain.
A fentanyl transdermal patch should be considered if severe pain is anticipated.
Sublingual or transmucosal buprenorphine may be a good alternative for cats with moderate pain.
Tramadol has some µ-opiate receptor affinity; however, its main effect is to inhibit serotonin and norepinephrine reuptake. This analgesic is commonly used after surgery in either species.
Future Follow-upPeriodontal aftercare includes home preventive maintenance (eg, daily brushing); institution of appropriate home care choices for enhancing plaque control, tartar, and gingivitis; and a schedule for return visits for periodontal care and prophylaxis.In GeneralRelative CostPain management for periodontal disease:Stage 0-1 = 0Stage 2 = $Stage 3 = $-$$$Stage 4 = $-$$$
Periodontal treatment:Stage 0-1 = $$-$$$Stage 2 = $$-$$$Stage 3 = $$-$$$$$Stage 4 = $$-$$$$$
Prognosis
The prognosis for periodontal disease for most patients is good providing the preceding follow-up recommendations are met, based upon the individual case.
Prognosis for pets with severe or refractory periodontitis is guarded-many pets resist home care because of chronic oral pain. Long-term therapy with opiates, corticosteroids, NSAIDs, and other compounds has been used historically and may be needed as an adjunct to the preceding periodontal aftercare recommendations to control oral pain in these cases.
Full mouth extractions are a viable option if home care and frequent prophylaxis are not possible because of pain or other reasons.
Adapted from Veterinary Dental Nomenclature, American Veterinary Dental College, www.avdc.org
PAIN MANAGEMENT & PERIODONTAL DISEASE • Brett BeckmanSuggested Reading
Alpha-2 agonists. Lamont L, Tranquilli W. In Gaynor JS, Muir WW (eds): Handbook of Veterinary Pain Management-St. Louis: Mosby, 2002, pp 199-220.Effects of morphine, lidocaine, ketamine, and morphine-lidocaine-ketamine drug combination on minimum alveolar concentration in dogs anesthetized with isoflurane. Muir WW, Wiese AJ, March PA. Am J Vet Res 64:1155-1160, 2003.Inflammatory mediators and modulators of pain. McMahon SB, Bennett LH, Bevan S, et al. In McMahon SB, Koltzenburg M (eds): Wall and Melzack's Textbook of Pain, 5th ed-China: Elsevier, 2006, pp 49-72.Pain behaviors. Muir WW, Gaynor JS. In Muir WW, Gaynor JS (eds): Handbook of Veterinary Pain Management-St. Louis: Mosby, 2002, pp 65-81.Pain management for oral surgery in dogs and cats. Beckman BW. J Vet Dent 23:50-60, 2006.Physiology and pathophysiology of pain. Muir WW. In Gaynor JS, Muir WW (eds): Handbook of Veterinary Pain Management-St. Louis: Mosby, 2002, pp 13-45.Postanesthetic hyperthermia in cats: A retrospective comparison between hydromorphone and buprenorphine. Niedfeldt R, Robertson S. Vet Anaesth Analg 33:381-389, 2006.Postoperative pain and its management. Dahl JB, Kehlet H. In McMahon SB, Koltzenburg M (eds): Wall and Melzack's Textbook of Pain, 5th ed-China: Elsevier, 2006, pp 635-651.Use of low doses of ketamine administered by constant rate infusion as an adjunct for postoperative analgesia in dogs. Wagner AE, Walton JA, Hellyer PW, et al. JAVMA 221, 72-75, 2002.Additional ResourcesAmerican Veterinary Dental Society:www.avdsonline.orgVeterinary Anesthesia & Analgesia Support Group: www.vasg.orgInternational Veterinary Academy of PainManagement: www.cvmbs.colostate.edu/ivapm