Oclacitinib
Elizabeth Falk, DVM, DACVD, Unleashed Veterinary Dermatology, Stratford, Connecticut
Lluís Ferrer, DVM, PhD, DECVD, Tufts Cummings School of Veterinary Medicine
Oclacitinib maleate is a novel oral tablet licensed for the control of pruritus associated with allergic dermatitis and the control of atopic dermatitis in dogs older than 1 year of age.1
Several studies have described the role of cytokine dysregulation in the development of both human and canine atopic dermatitis (CAD).2,3 Specifically, there is evidence of T-helper type 2 (Th2) and type 1 (Th1) imbalance with increased expression of Th2 cytokines (IL2, 4, 6, 13) found in lesional and nonlesional skin of atopic dogs.4,5 Janus kinases (JAKs) play a crucial role in cytokine signaling, with JAK1 and JAK3 involved in the signaling of numerous cytokines involved in allergy and inflammation (IL-2, 4, 6, and 13), including IL-31, which is considered to have a major role in the physiopathology of canine pruritus.6
As a specific JAK1 (and, to a lesser degree, JAK3) inhibitor, oclacitinib is designed to inhibit proinflammatory and proallergic cytokines (IL-2, 4, 6, 13, 31) involved in the pathogenesis of atopic dermatitis, thereby helping to reduce pruritus and inflammation.6,7 Oclacitinib has little effect on cytokines involved in hematopoiesis that are dependent on JAK2.6,7
Sounding Board
Indications
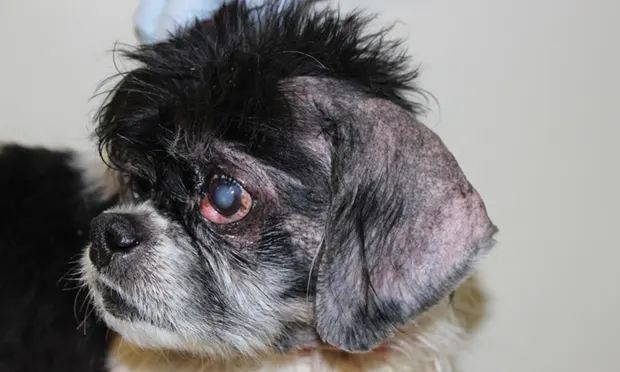
Oclacitinib has been shown to be effective in the treatment of nonspecific allergic dermatitis and atopic dermatitis in dogs (Figures 1 and 2).8,9 The recommended dose is 0.4-0.6 mg/kg PO twice daily, administered for the first 2 weeks, followed by PO 0.4-0.6 mg/kg once daily as a maintenance dose.7,9 Long-term twice-daily administration is not recommended.

Severe atopic dermatitis before treatment with oclacitinib. Note marked erythema and hyperpigmentation on the abdomen, axillae, and face.

Two months after treatment with oclacitinib.
The drug is currently not licensed for use in cats; however, a recent study has reported the extralabel use of oclacitinib in cats with nonflea- and non-food-induced hypersensitivity dermatitis at a dose of 0.4-0.6 mg/kg twice a day for 2 weeks, followed by 2 more weeks at the same dose once a day. The drug at this dose seemed to be safe, but the efficacy was scored as good or excellent only in 4 of the 12 cats. The authors conclude that further studies are needed to identify the most effective dose and regimen for this species.10
When compared with other treatment modalities of CAD, oclacitinib is demonstrated to be as effective as prednisolone (0.5-1 mg/kg once a day for 6 days, then once every 2 days for 28 days).10 A recent study demonstrated comparable efficacy to cyclosporine at 3.2-6.6 mg/kg once a day for controlling the pruritus and inflammation of CAD.11
Potential Indications
There are some emerging extralabel indications under investigation in clinical settings with no published data regarding their efficacy. Specifically, future indications may involve oclacitinib in the management of other pruritic dermatoses (eg, temporary treatment of pruritus in scabies patients), immune-mediated dermatologic conditions (eg, canine perianal fistulas, pemphigus foliaceus), or reactive dermatoses (eg, reactive istiocytosis).12
Although oclacitinib is not labeled for use in cats, 1 study reports oclacitinib being used to treat cutaneous mastocytosis in a cat (1 mg/kg twice a day for 31 days).13
Contraindications
As an immunomodulatory agent, oclacitinib is contraindicated in dogs with a history of neoplasia, generalized demodicosis, and concurrent serious infection.7,9 It is not labeled for use in breeding animals. Oclacitinib is not labeled to be used in dogs younger than 1 year of age, as a few dogs in this age range developed adverse events (eg, pneumonia, demodicosis) in original field studies.14 The safety of the concurrent use of oclacitinib with other immunosuppressive or immunomodulatory drugs (ie, corticosteroids, cyclosporine) has not been formally evaluated and is not recommended.
Because of low plasma-protein binding and minimal inhibition of cytochrome P450, the risk for drug–drug interactions is believed to be low.7,9
Advantages
Advantages of oclacitinib include:
Works rapidly and effectively.
Well-tolerated and easy to use. Has fewer GI effects than modified cyclosporine, a recommended treatment for CAD.11
Has low plasma protein binding and minimal inhibition of cytochrome P450, possibly minimizing the risk of drug–drug interactions.7
Does not interfere with allergy testing (serum-specific IgE testing or intradermal allergy testing).9
Can be used in combination with immunotherapy during initial phases or can be used as an adjunct for flares of atopic dermatitis.
Disadvantages
Disadvantages of oclacitinib include:
Short-duration studies suggest a positive safety profile, but little is known about long-term safety. One study has demonstrated a good long-term safety profile of more than 600 days.16
Increased pruritus can occur in some dogs transitioning from the twice-a-day loading dose to the once-a-day maintenance dose. This can be temporary and resolve spontaneously. In persistent cases in the authors’ experience, moving the administration of the tablet to the most pruritic part of the day can help restore control of the pruritus.
Cannot be used in combination with steroids.
Adverse Effects
Clinical trials have suggested that oclacitinib is safe in the short term when used at recommended doses in the dog.8,9,11,12,15 In several studies, a small percentage of dogs on daily treatment (<5%; similar to the placebo group) developed diarrhea or vomiting.8,9 In all reported studies, a small but consistent number of dogs (<10%) on daily treatment with oclacitinib developed benign skin neoplasia (histiocytomas, among others).8,15 In a recent study, urinary tract infection and cystitis (11.3%), vomiting (10.1%), otitis (9.3%), pyoderma (9.3%), and diarrhea (6.1%) were the most frequently reported abnormal clinical signs.16
CAD = canine atopic dermatitis, JAKs = janus kinases
*Note: Dr. Falk’s dermatology residency program receives partial funding from Zoetis.