Neurologic Signs in a Dog with Demodicosis
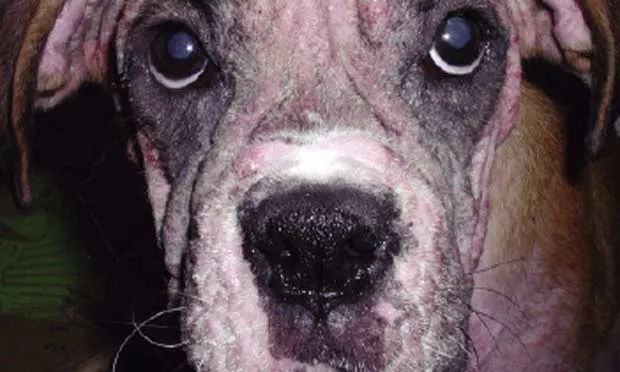
A 2-year-old castrated male boxer was presented for tremors and incoordination.
HistoryTremors and incoordination occurred about 5 hours after the dog received a single dose (30 mg/kg PO) of the flea preventive spinosad (Comfortis, elanco.com).
The dog had been diagnosed with young-onset generalized demodicosis and secondary superficial pyoderma 3 months earlier. At that time, treatment was initiated with ivermectin 1% (0.6 mg/kg PO Q 24 H), cephalexin (30 mg/kg PO Q 12 H), and benzoyl peroxide shampoo (Pyoben, virbacvet.com) twice weekly. He responded well to treatment, and because the superficial pyoderma resolved 2 months after cephalexin treatment was initiated, that drug was discontinued.
Physical ExaminationThe dog was lethargic and ataxic and had bilateral mydriasis. There was mild generalized alopecia but evidence of hair regrowth. No other physical abnormalities were noted.
Laboratory ResultsComplete blood count and serum biochemical profile results were unremarkable. Skin scrapings revealed a few Demodex mites.
Ask Yourself...1. What is the most likely cause of this dog’s neurologic signs?A. Ivermectin toxicityB. Spinosad toxicityC. Drug interaction toxicity (ivermectin & spinosad)D. Unrelated to prescribed medications
2. How would you manage the neurologic signs?A. Provide supportive careB. Discontinue ivermectinC. Discontinue spinosadD. A, B, and C
**CORRECT ANSWERS:1C. Drug interaction toxicity (ivermectin & spinosad)2D. A, B, & C
**Ivermectin and spinosad were discontinued, and the owner was instructed to provide supportive care until clinical signs resolved.
IvermectinIvermectin is not approved by the U.S. Food and Drug Administration (FDA) for treatment of canine demodicosis; however, daily oral administration of extralabel high doses (0.4–0.6 mg/kg Q 24 H) of the drug is commonly used for this purpose. The most common causes of ivermectin toxicity are related to administration of high doses (10–20 times the recommended dose) and breed sensitivity. Signs of ivermectin toxicity include lethargy, tremors, vomiting, drooling, ataxia, mydriasis, stupor, and coma; this toxicity may result in death.
Signs of Ivermectin Toxicity
Ataxia
Drooling
Lethargy
Mydriasis
Tremors
Vomiting
Coma
Death
Stupor
SpinosadSpinosad is used orally on a monthly basis for prevention and treatment of flea infestations. The most common side effect associated with spinosad is vomiting, but lethargy, diarrhea, cough, increased thirst, vocalization, increased or decreased appetite, erythema, hyperactivity, and excessive salivation may also be noted. No neurologic signs have been reported in open-label dose response clinical trials for spinosad.
Drug InteractionThe neurologic signs presented in this case are consistent with ivermectin toxicity; however, neurotoxic effects of ivermectin used alone usually occur during the first few days or weeks of therapy. In addition, the neurologic signs occurred shortly after spinosad administration in this case. Therefore, the lack of neurologic signs during the first 3 months of ivermectin therapy and the sudden onset of neurologic signs after spinosad administration suggest that in this dog the signs were likely due to a drug interaction.
TreatmentDiscontinuation of ivermectin and spinosad therapy, along with symptomatic and supportive care, is important for recovery. An antidote for ivermectin toxicity (physostigmine) may be used if needed. Once the dog recovers, demodicosis drug treatment and flea preventive should be carefully reinitiated avoiding the use of the 2 drugs concurrently or using alternative medications.
FDA & Manufacturer RecommendationsOn June 24, 2008, the FDA Center for Veterinary Medicine and Elanco Companion Animal Health reported adverse reactions consistent with ivermectin toxicity in dogs receiving ivermectin and spinosad concurrently. Veterinarians were advised that dogs receiving extralabel high doses of ivermectin should not receive concurrent treatment with spinosad.
According to the manufacturer, concurrent administration of spinosad and approved canine ivermectin and milbemycin oxime at doses labeled for heartworm prevention has been tested and shown to be safe, even when used in dogs carrying the ABCB1 (previously MDR1) gene mutation that causes avermectin sensitivity.
Potential PathophysiologyTo date, concurrent administration of extralabel doses of ivermectin and spinosad has not been evaluated in laboratory or field studies. Ivermectin is an agonist for the neurotransmitter gammaaminobutyric acid (GABA). Although spinosad has a novel mode of action, primarily targeting nicotinic acetylcholine receptors, it also has secondary effects on GABA neurotransmission. The effect of both drugs on GABA has been speculated as a possible cause of the observed ivermectin neurotoxicity; however, inhibition of P-glycoprotein, which would increase ivermectin access to the central nervous system, has also been proposed as a potential explanation for the response.1
Take-Home Messages
Ivermectin for the treatment of canine demodicosis is extralabel and requires careful vigilance for unexpected reactions, mainly with concurrent administration of other drugs.
Use of spinosad in dogs receiving extralabel high doses of ivermectin should be avoided.
Spinosad is approved and safe for use with labeled doses of heartworm preventives.
Discontinuation of ivermectin and spinosad, along with supportivetherapy, should lead to complete cessation of neurotoxic effects.