Neurologic Examination
Carolyn Nye, DVM, Massachusetts Veterinary Referral Hospital, Woburn, Massachusetts
Mark Troxel, DVM, DACVIM (Neurology), Massachusetts Veterinary Referral Hospital, Woburn, Massachusetts
There are 5 main sections of the neurologic examination: mental status, gait analysis and body posture, cranial nerves, postural reactions, and spinal and withdrawal reflexes.
There are several additional tests that do not fall into these categories, such as assessment of muscle tone and muscle atrophy, perineal reflex, and cutaneus trunci reflex and evaluation for nociception and neck and/or back pain. As with a general physical examination, the neurologic examination should be conducted the same way every time to develop a routine and prevent errors. Using a neurologic examination form is strongly recommended (see Neurologic Examination Form).
Mental Status
The patient’s mental status should be assessed during history-taking and throughout the appointment. The patient should be alert and responsive at all times. The patient’s interactions with humans and its environment should be noted, including:
Whether the patient is fully alert and responsive
Whether the patient has difficulty navigating corners or tight spaces
Whether the patient walks in circles
Whether the patient responds to its name
Gait Analysis & Body Posture
Analysis of gait and body posture early in the examination is especially important in cats that are nervous or stressed, as they may refuse to walk later if gait is analyzed after the hands-on portion.

Gait analysis should be performed on a surface with adequate traction. The patient should be evaluated while walking toward and away from the observer, as well as in profile.
Dogs should be walked on a short leash away from, toward, and in profile to the observer (Figure 1), ideally on a surface with good traction (eg, concrete, grass, nonslip floors). The gait should be observed for signs of weakness (paresis) or paralysis (plegia), ataxia, and lameness. When describing the gait, first identify whether the patient is ambulatory or nonambulatory, then identify which limbs are affected and the degree of weakness (Table). For instance, a dog with nonambulatory paraparesis is too weak to walk on its own but retains voluntary motor function in the pelvic limbs and is normal in the thoracic limbs.
Table: Keywords: Gait Analysis
Example: A patient with one limb affected with weakness would have monoparesis.
The patient should be evaluated for ataxia (ie, incoordination). There are 3 types of ataxia: proprioceptive, cerebellar, and vestibular. Proprioceptive ataxia is characterized by scuffing or dragging the paws on the ground, knuckling over, crossing over, or interference (ie, limbs hitting each other when walking). Vestibular ataxia is characterized by leaning or falling to one side. Patients with vestibular ataxia often use a wall for support while walking. Cerebellar ataxia is characterized by dysmetria (ie, unequal range and force of each step), often with hypermetria (ie, overflexion of the elbows in the thoracic limbs or hips/stifles in the pelvic limbs). Patients will sometimes have an overreaching, long-strided, floating gait, especially in the thoracic limbs, that can look like cerebellar hypermetria. However, this type of gait is caused by an upper motor neuron (UMN) lesion. The key difference between cerebellar hypermetria is overflexion of the elbow as compared with straight elbows in the overreaching UMN gait. Finally, any postural abnormalities (eg, head tilt, head turn, torticollis) should be noted.
Author Insight
Dogs and cats with a two-engine gait have a short and choppy thoracic limb gait and a long-strided and floating pelvic limb gait. A short, choppy gait is a sign of lower motor neuron dysfunction, whereas a long-strided/overreaching gait is a sign of upper motor neuron dysfunction. This combination of gait alterations is suggestive of a C6-T2 myelopathy. A short, choppy gait may also be observed with neuromuscular or orthopedic disease (eg, bilateral elbow osteoarthritis).
Cranial Nerve Examination
The hands-on examination should take place after gait and mentation are evaluated. Most neurologists start with the cranial nerves (CNs), either by testing the CNs in numerical order or by location (eg, performing all eye-related tests then evaluating nose/face, ears, mouth). There are 12 pairs of CNs, but not all are routinely tested as part of the neurologic examination.
Cranial Nerves Tested in Neurologic Examination
I. Olfactory: Sense of smell; not routinely tested
II. Optic: Vision
III. Oculomotor: Motor innervation of extraocular muscles (ie, dorsal, medial and ventral rectus, ventral oblique) and the levator palpebrae dorsalis; parasympathetic supply for pupil constriction and lens accommodation
IV. Trochlear: Motor innervation of dorsal oblique muscle
V. Trigeminal: Sensory to all of face except inner pinnae; motor to muscles of mastication
VI. Abducens: Motor to lateral rectus and retractor bulbi muscles
VII. Facial: Motor to muscles of facial expression and caudal belly of digastricus; parasympathetic supply for lacrimation; sensory for taste to rostral two-thirds of tongue; sensory to inner pinnae and external auditory canal
VIII. Vestibulocochlear: Balance and equilibrium (vestibular); hearing (not routinely tested during neurologic examination)
IX. Glossopharyngeal: Mastication; deglutition; respiration; vocalization; parasympathetic to parotid and zygomatic salivary glands; taste from caudal one-third of tongue; sensory for gag reflex, baroreceptor reflex, O2 and CO2 receptors
X. Vagus: Parasympathetic to thoracic and abdominal viscera and heart; deglutition; respiration; vocalization; skin of external auditory meatus and canal
XI. Accessory: Motor to the trapezius, cleidocervicalis, sternocephalicus, and omotransversarius muscles; not routinely tested
XII. Hypoglossal: Motor to intrinsic tongue muscles
Step-by-Step: Cranial Nerve Examination
What You Will Need
Reflex hammer
Hemostats
Strong light source (eg, transilluminator, halogen penlight)
Lens (direct or indirect)

Step 1
The menace response (Figure 2) is the first step of the CN examination and tests CNs II and VII. Cover one eye, then bring the hand toward the other eye in a “menacing” gesture. Be careful not to generate wind current or touch any whiskers or eyelashes, as it is important not to let the patient feel the hand’s presence. It may be necessary to lightly tap the animal’s head to get its attention. The animal should blink. Perform the same steps in the other eye.

Care should be taken to prevent air currents or touching the whiskers or eyelashes when performing the menace response test. The authors prefer an “explode-the-hand” technique.
Author Insight
The menace response is an age-related learned response—not a reflex. Dogs and cats typically develop the menace response by 10 to 12 weeks of age; thus, dogs and cats younger than 12 weeks of age may not have a menace response.2
The cerebellum exerts some influence over the menace response, but the exact mechanism is unclear. Patients with cerebellar dysfunction will sometimes have an absent menace response ipsilateral to the lesion. Assuming no other intracranial lesions are present, these patients would have normal vision, pupillary light reflexes, and palpebral reflexes.2
Step 2
Test the pupillary light reflex to test cranial nerves II and III. Use a strong, focused light source (eg, transilluminator, halogen penlight).
Step 3
Perform a fundic examination. The optic disc is the only part of the CNS that can be directly evaluated on clinical neurologic examination. Retinal abnormalities also provide clues to systemic health. Either a direct (ophthalmoscope) or an indirect (lens and transilluminator) fundic examination can be performed, but the authors prefer an indirect examination so that the observer’s face is not immediately next to the patient’s mouth.
Step 4
Test the palpebral (blink) reflex by lightly tapping the medial and lateral canthus of both eyes to assess the patient’s ability to blink, which tests CNs V and VII. If possible, approach the eyes from behind the head so the patient cannot see the instrument approaching and give a false-positive result. Test corneal sensation (CNs V and VII) and globe retraction (CNs V and VI) by gently touching the cornea with a sterile cotton swab. The normal response includes eyelid closure and eyeball retraction. Corneal sensation and globe retraction are typically tested only when other CN abnormalities are noted, as all 3 of the involved CNs (V, VI, and VII) in these reflexes can be tested by other means; for example, sensation (V) can be tested by stimulating other regions of the face, and facial nerve (VII) function can be tested through menace response and palpebral reflex.
Step 5
Evaluate for physiologic nystagmus (ie, “doll’s eye”) by turning the animal’s head from side to side and up and down. This should induce a normal nystagmus with a fast phase in the direction of head movement. Determine whether the patient has abnormal (pathologic) nystagmus at rest (ie, neutral body position) and in different body positions (eg, on side or back). Abnormal nystagmus is common with vestibular dysfunction. Pathologic nystagmus should be characterized by the direction of the fast phase and whether it is horizontal, rotary, or vertical. Evaluate CNs III, IV, and VI by looking for strabismus or abnormal eye movements.
Step 6
Palpate the patient’s head to assess for pain or atrophy of the muscles of mastication. Asymmetric atrophy of the masticatory muscles is suggestive of disease of ipsilateral trigeminal nerve (most often trigeminal nerve sheath tumor), whereas bilateral atrophy with difficulty opening the jaw is most common with masticatory muscle myositis.
Step 7
Stimulate (via touch or pinch) different areas of the head to assess sensation (CNs V [all over face] and VII [inner surface of pinnae]) and motor response (CN VII).
Step 8
Using a small blunt instrument, touch the nasal mucosa just inside the nose. The patient should pull its head away. This tests CN V and the contralateral cerebral cortex as the patient interprets the stimulus and pulls its head away.
Step 9
Open the patient’s mouth and assess the degree of jaw tone (CN V). Evaluate the tongue for deviation, atrophy, or fasciculation (CN XII). For a cooperative dog, perform a gag reflex (CNs IX and X) by gently touching the oropharynx or laryngopharynx. The dog should swallow. Do not perform a gag reflex in aggressive dogs, nervous dogs, or any cat. Instead, lightly palpate the laryngeal region externally to induce swallowing.
Author Insight
Vertical nystagmus is suggestive of central vestibular dysfunction but should be interpreted cautiously, as some patients may appear to have vertical nystagmus when there is actually a slight rotational component. Do not rely on vertical nystagmus as the sole indicator of central vestibular dysfunction.
The gag reflex can be unreliable, as some normal dogs will not swallow. A history of gagging, retching, or coughing while eating or drinking is more reliable.
There is no test to evaluate CN XI, but animals with a CN XI deficiency may have atrophy of the trapezius, cleidocervicalis, sternocephalicus, and/or omotransversarius muscles, although atrophy of these muscles is difficult to appreciate clinically.
The authors do not routinely test olfaction, taste, or hearing, as results can be subjective in animals, and it can be difficult to determine if there is partial loss of these sensations.
Postural Reactions
Next is evaluation of postural reactions. The authors recommend that several postural reaction tests be performed, as subtle abnormalities may not be detected on general proprioception, which typically includes paw replacement, “knuckling,” and conscious proprioception).
Step-by-Step: Postural Reactions
Step 1
Measure general proprioception (Figure 3) in all limbs. Support the patient’s trunk (for the thoracic limbs) and below the abdomen (for the pelvic limbs) so that it is minimally weight-bearing on the limb but maintains good contact with the ground.

General proprioception (paw replacement, knuckling) is best performed by gently manipulating only the paw. Larger limb movements stimulate proprioceptors in the joints and muscles.
Step 2
One at a time, turn each paw over so that the dorsum of the paw is on the ground. The patient should immediately return the paw to a normal position. Watch for delays in replacement or abnormal placement.
Step 3
Perform at least one additional test to assess proprioception, as some patients will have normal general proprioception but will show abnormalities on the other tests. Do not rely solely on general proprioception, as failure to return the paw to normal position can result from use of a muzzle or the owner distracting the dog through petting. These additional tests include hopping (ie, lifting the patient off the ground to hop on one leg laterally), wheel-barrowing (ie, lifting the pelvic limbs off the ground and pushing the patient forward on its thoracic limbs), and extensor postural thrust (ie, lifting the patient off the ground and bringing the pelvic limbs to the ground with the patient walking backward). The normal reaction is a swift, normal placement of the limb(s) in the direction of movement. Visual and tactile placing can be performed in cats and small dogs. In cats, it is often easier to perform these proprioceptive tests than it is to perform general proprioception, although some cats refuse to cooperate.
Spinal Nerve Reflexes
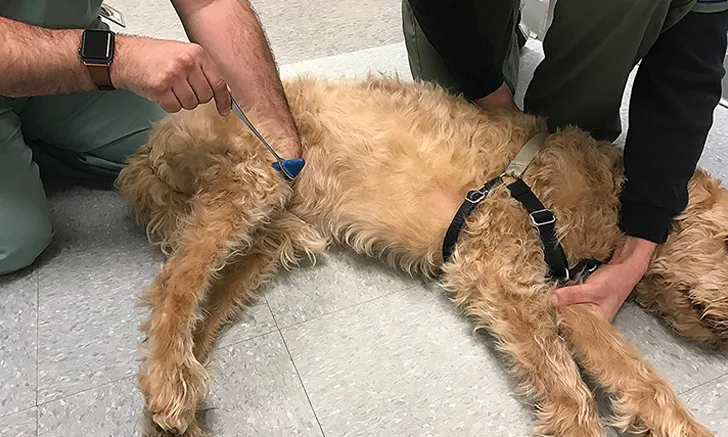
Ideally, reflexes should be tested in lateral recumbency, but they can also be tested in standing (uncooperative) patients. The most reliable reflexes are the patellar and withdrawal (flexor) reflexes. Other reflexes (eg, cranial tibial, extensor carpi radialis) are less reliable and commonly produce false-negative results. The patellar reflex (Figure 4) can be used to evaluate the femoral nerve.

The patellar reflex is the most reliable stretch reflex. Gently hold the limb off the ground with the stifle slightly flexed, then strike the straight patellar tendon with a reflex hammer.
Patellar Reflex
To perform the patellar reflex, hold the pelvic limb up, with the knee slightly flexed, and strike the straight patellar tendon with a reflex hammer. The patient should extend the stifle.
Withdrawal Reflex
Pinch the webbing between the toes. A complete withdrawal reflex (Figure 5) involves fully flexing all joints in the limb. If the patient does not respond, use hemostats with the least amount of pressure necessary to induce flexion. The withdrawal reflex tests a combination of nerves (ie, median, ulnar, musculocutaneous, axillary, radial) in the thoracic limbs and the sciatic nerve in the pelvic limbs, although the femoral nerve is involved in hip flexion.

During withdrawal reflex testing, the patient should flex all joints of the limbs. Use the least amount of pressure needed to elicit a response. A hemostat should only be used if a withdrawal reflex cannot be elicited using the fingertips.
Of note, limb withdrawal is a local spinal reflex and not a sign of intact pain perception, which requires a conscious response (eg, crying, whining, attempting to bite).
Author Insight
Crossed extension involves extension of the contralateral limb while the toes of one limb are pinched. This is an abnormal response in a recumbent animal and is indicative of a UMN lesion. This may occur in some nervous or stressed animals, so crossed extension should be interpreted cautiously.
In general, lesions in the L4-S2 region of the spinal cord cause decreased patellar and withdrawal reflexes in the pelvic limbs. Increased reflexes typically indicate a UMN disorder. An exaggerated patellar reflex may be observed with peripheral sciatic nerve dysfunction or with a lesion in the L6-S2 region of the spinal cord where the sciatic nerve originates if the femoral nerve (L4-6) is unaffected. Sciatic nerve dysfunction can lead to decreased muscle tone in the antagonistic stifle flexor muscles. Because the opposing force is reduced by sciatic nerve dysfunction, the patellar reflex may become exaggerated (ie, pseudohyperreflexia).
Ancillary Tests
Additional tests include evaluation of muscle tone and atrophy, cutaneus trunci reflex, perineal reflex or anal tone, nociception, and neck or back pain. Muscle tone and atrophy of the trunk and limbs are most often tested with the patient standing in a normal position but can also be evaluated with the patient in lateral recumbency.
Cutaneus Trunci Reflex
To test the cutaneus trunci reflex, lightly pinch the skin with hemostats on either side of midline starting at L6 and moving cranially. The skin should flinch bilaterally as the cutaneus trunci muscle is stimulated. Once a normal response is elicited, there is no need to continue further cranially. Cutaneous sensory fibers enter the spinal cord via segmental spinal nerves, and some cross over immediately in the spinal cord and ascend bilaterally to synapse on cell bodies, giving rise to the lateral thoracic nerve (C8-T1). Because sensory fibers ascend the spinal cord bilaterally, a skin twitch should occur on both sides, regardless of the side pinched. Animals with a spinal cord lesion may have an absent cutaneus trunci reflex (ie, cut-off) that occurs approximately 2 to 3 segments (range, 0-4) caudal to the spinal cord lesion.1 Patients with lateral thoracic nerve dysfunction (eg, brachial plexus injury, nerve sheath tumor) may have an absent cutaneus trunci reflex ipsilateral to the lesion, regardless of which side is pinched.
Near the end of the examination, a hemostat can be used to lightly stimulate the perianal area. The external anal sphincter should constrict reflexively. Digital rectal examination may be required to further evaluate anal tone.
Back & Neck Pain Test
Testing for back or neck pain and nociception (ie, conscious perception of a noxious stimulus) should be conducted at the end of the examination so that it does not alter the results of the rest of the neurologic examination.
Palpate firmly down the length of the vertebral column, then test for range of motion of the neck and response to elevation of the tail. Nociception should only be tested in affected limbs that are paralyzed. Although there are exceptions, if a patient can voluntarily move the limb at all, nociception in that limb should be intact. Nociception should be evaluated in the affected limb(s) by pinching the digits (lateral and medial toes) with a hemostat. Nociception should also be tested in the tail. The patient should have a visible response to the stimulus, such as turning around to look, crying, whining, biting, or experiencing a change in respiratory rate to indicate perception of the noxious stimulus. As stated above, flexion of the limb is a local reflex and not a sign of conscious perception.
Conclusion
Clinical neurologic signs depend on lesion location rather than etiology. Thus, the goal of the neurologic examination is to determine the affected area of the nervous system first and, from there, formulate a list of possible differentials. After this, a diagnostic and therapeutic plan can be developed.
CN = cranial nerve, UMN = upper motor neuron
The authors would like to thank Jillian Marvelley, CVT, and Bill Noonan for their assistance with the neurologic examination images and the Pinheiro family for allowing Simba to be the model.