Nasoesophageal & Nasogastric Tube Placement
Kathleen Aicher, DVM, North Carolina State University
Brian Allett, DVM, University of Tennessee
Karen M. Tobias, DVM, MS, DACVS, University of Tennessee
Early feeding of hospitalized veterinary patients is critical for prevention of malnutrition and recovery from systemic illness. Enteral nutrition may be preferred to parenteral nutrition when there is adequate GI tract function. Enteral nutrition helps maintain the structure and function of the GI tract and acts as an immunologic barrier. Recovery from such conditions as parvoviral enteritis is faster with enteral nutrition.1 Patients should be given a chance to eat on their own before tube feeding is initiated.
Enteral nutrition is commonly delivered via a nasoesophageal (NE), nasogastric (NG), esophagostomy, gastrostomy, or enterostomy tube. NE and NG feeding tubes are relatively inexpensive, can be placed quickly and easily, do not require general anesthesia, and are generally well tolerated by cats and dogs (Figure 1). The small tube size allows patients to eat or drink around the tubes. Although NE and NG tubes are normally used for short-term feeding, they can be retained for several weeks2; however, unlike gastrostomy and enterostomy tubes, NE and NG tubes can be removed within hours of placement. Evidence that NG tubes can cause signs of reflux esophagitis is lacking (possibly because of their small size), despite the fact that they cross the lower esophageal sphincter.3,4

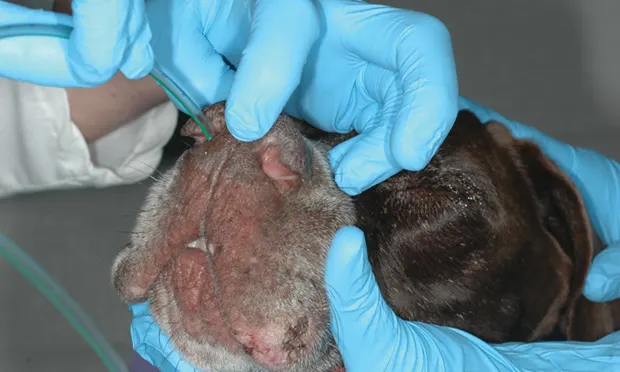
Although Elizabethan collars are recommended, many patients do not tamper with NE tubes if they are secured to the patient in a comfortable location. In this dog, the NE tube is sutured to the skin caudolateral to the planum and on the forehead.
Care & Considerations
The small internal diameter of NE and NG tubes is a major limitation. When a 5- or 6-French tube is placed, supplementation is limited to a liquid enteral diet. If they can be used, larger tubes can accommodate a diluted, commercially homogenized recovery diet. Liquid diets can be delivered via constant-rate infusion (CRI) or intermittent bolus (see Developing a Feeding Plan). If a syringe pump is used for CRI, the fluid line should be changed daily to prevent clogging.
Developing a Feeding Plan
Calculate resting energy requirement (RER) for the patient’s current or ideal weight (if obese).
For accuracy, use the formula: RER (kcal/day) = 70 × [body weight (kg)]0.75
RER may need to be adjusted to address underlying disease and is often multiplied by a factor of 1.25 to meet increased caloric requirements.
If the patient is neither drinking nor on IV fluids, calculate fluid requirements.
Daily mL of required water is approximately the same number as the daily RER kcal.
Feed one-fourth to one-third of nutritional requirements on day 1; deliver via CRI or in 6–8 feedings.
If there is risk for refeeding syndrome (see Author Insight), check electrolytes within 24 hours of initial feedings.
Gradually increase volume and decrease frequency of feeding so the patient is receiving 100% of nutritional requirements in 3–4 feedings, or with CRI, by day 3 or 4.
If bolus delivery is elected, the tube should be aspirated between feedings to confirm tube patency, GI motility, and adequate passage of the previous meal. If more than 20% of the previous meal is aspirated, prokinetic drug administration and reduced food intake are recommended. The diet should be warmed to near body temperature and slowly administered over 10 to 15 minutes. If vomiting, hypersalivation, or abdominal distention occurs during infusion, the volume and infusion rate should be decreased and feeding frequency increased. To prevent occlusion between feedings, the tube should be flushed and capped with a column of water remaining.
Contraindications & Complications
NE and NG tubes should not be placed in patients that are comatose, laterally recumbent, or dyspneic; lack a gag reflex; or have esophageal dysfunction or obstruction. Tubes should be placed with caution in patients with thrombocytopenia or coagulopathies to avoid uncontrollable nasal bleeding. Although vomiting sometimes contraindicates their use, NE and NG tubes have been successfully placed in vomiting patients; vomiting may be significantly reduced with NE or NG tube feeding.1,3
Potential complications of NE and NG tube placement and feeding include epistaxis, hypersalivation, vomiting, diarrhea, dacryocystitis, tube obstruction, or inadvertent tube dislodgement.
Related Article: How To Place An Esophagostomy Tube
Related Article: Feeding the Critically Ill, Anorectic Dog
Evaluating Tube Placement
Tubes can be accidentally misplaced in the trachea (Figure 2A), nasopharynx, or nasal cavity, and subsequent feeding may result in aspiration pneumonia; therefore, appropriate placement should be confirmed before feeding is initiated. Various inexpensive methods can help verify tube location: laryngoscopic visualization of the tube entering the esophagus; suction to verify negative pressure; air infusion while auscultating the abdomen for borborygmus; infusion of sterile saline or nonionic contrast medium (Figure 2B), which sometimes elicits a cough with tracheal placement; or pH assessment of fluid aspirated from the tube.


Inadvertent placement of an NG tube in the trachea and bronchus of a miniature dachshund (11 years of age). This dog had a severe pulmonary interstitial pattern secondary to infusion of 20 mL of sterile saline through the tube; it had no cough reflex during infusion (A). After injection of 3 mL of iohexol 240, positive contrast medium outlined the alveoli, particularly in the caudodorsal thorax, confirming tube misplacement (B). The following day, radiography confirmed that contrast medium and saline had been cleared from the lungs.
Another quick way to assess placement is to attach the tube end to an airway gas analyzer (eg, capnograph). When the tube end is located in the esophagus or stomach, there is no capnographic waveform, respiratory rate, or end-tidal CO2 detectable by the machine, which sets off its apnea alarm.6 End-tidal CO2 and respiratory rate are both measureable, and a capnographic wave-form is elicited if the tube end is in the trachea, nasopharynx, or nasal cavity. Because an NE or NG tube could be coiled in the nasopharynx (Figure 3) or kinked and still terminate in the esophagus or stomach, survey or contrast radiography is recommended to verify placement. Use of a hyperosmolar, ionic iodinated contrast medium (eg, diatrizoic acid [Hypaque]) should be avoided, as its use in a tube improperly placed in the lungs can result in inflammatory reactions and pulmonary edema.

Although this tube terminates in the esophagus, tube coiling in the nasopharynx and oropharynx will likely cause coughing or vomiting and subsequent oral expulsion of the tube end.
Step-by-Step: Nasoesophageal or Nasogastric Feeding Tube Placement
What You Will Need
Sedative ± analgesic (eg, opioid) and local anesthetic
0.5% proparacaine hydrochloride or 2% lidocaine hydrochloride solution
Water-based lubricant or 2% lidocaine gel
5-, 6-, or 8-French radiopaque polyurethane or silicone elastomer tube
Skin marker
1-inch, white tape
Skin stapler or 3-0 nylon suture on a straight needle
Super or tissue glue (optional)
6-mL syringe
3–5 mL of sterile saline
Elizabethan collar
Nonionic radiographic contrast medium (eg, iohexol) or end-tidal CO2 monitor
Step 1
If the patient will be difficult to restrain during suture or staple placement (see Step 5), sedate before starting. Measure the distance from the tip of the patient’s nose to the seventh or eighth intercostal space for placement of an NE tube (shown) or to the last rib for an NG tube. Mark the tube at the appropriate length. To determine when to expect the patient to swallow the tube, measure and mark the distance from nose tip to hyoid apparatus.
Author Insight
Anorectic patients receiving enteral feedings should be monitored for dramatic decreases in phosphate, magnesium, and potassium levels, which are clinicopathologic abnormalities associated with a rare metabolic disorder known as refeeding syndrome. Refeeding syndrome can result in hematologic, neuromuscular, neurologic, pulmonary, and cardiovascular complications and death.

Step 2
Tilt the patient’s head upward and infuse 4–5 drops of 5% proparacaine hydrochloride or 2% lidocaine hydrochloride solution into each nostril. Wait several minutes for the local anesthetic to take effect. While waiting, lubricate the tube tip with a water-soluble or 5% lidocaine gel.
Author Insight
Premedicating both nostrils allows immediate passage into either of the nares once the anesthetic has taken effect.

Step 3
Place the patient in a standing, seated, or sternally recumbent position with the neck slightly extended and the head in a neutral position. For dogs, push the nasal planum upward (A), and insert the tube up and over the ventral ridge at the proximal end of the nasal passage. Direct the tube ventromedially through the nostril into the ventral meatus. Quickly advance the tube into the nasal cavity. If the dog pulls away, release the tube immediately to prevent extraction.
In cats, insert the tube in the medial aspect of either of the nares (B), and direct it caudoventrally.


Step 4
Advance the tube steadily into the nasopharynx. If there is resistance, withdraw the tube and redirect it ventrally to avoid the ethmoid turbinates. Hold the head in a normal position and allow the patient to swallow the tube.

Sagittal CT image of the nasal cavity of a dog. Arrowheads illustrate the path the tube must take. The tube must pass over the incisive bone and roots of the incisors before it can be directed through the ventral meatus, ventral to the ventral nasal concha (arrow) and ethmoid turbinates.
Step 5
Once the tube is advanced to the appropriate mark, secure the remainder to the patient’s head.
In dogs, use suture (± tissue glue) or skin staples to secure the tube near or along the nasal planum (A). At a point level with the forehead (dogs or cats) or the buccal pouch on the lateral face (dogs), place butterfly tape on the tube and staple or suture the tape to the skin (B). Place an Elizabethan collar and secure the tube on the neck under and behind the collar.
Author Insight
If a finger trap pattern is used, several drops of tissue glue can be placed along the suture to prevent slippage when the tube becomes wet (A).


Step 6
Confirm initial tube placement by suctioning to verify presence of negative pressure, flushing with 3–5 mL of sterile saline and listening for a cough, or attaching the tube to an end-tidal CO2 monitor.
Verify tube location with survey (A) or contrast (B) radiography. Inject 2–3 mL of iohexol or other nonionic, iodinated contrast medium into the tube, followed by 3–5 mL of air or sterile saline. Note how the contrast medium highlights the esophageal folds.
Author Insight
When an NE tube is placed appropriately, its end is located at the eighth intercostal space (approximately). Even properly placed tubes can appear to be in the airways, because the esophagus overlies the trachea and bronchi on caudal cervical and thoracic radiographs.


In this dog, the proximal end of the tube is not associated with the trachea.