Lymph Node Cytology
Anne Barger, DVM, MS, DACVP, University of Illinois
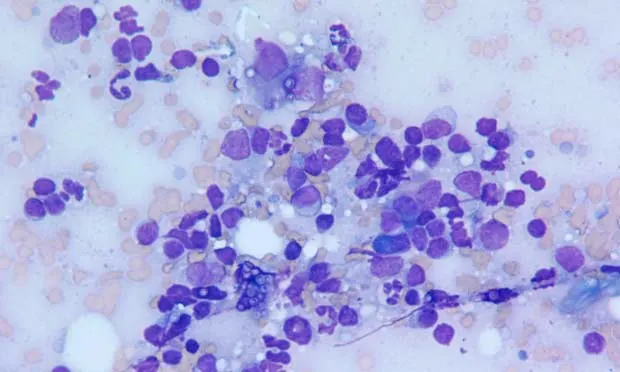
A 2-year-old mixed-breed dog presented with a several-week history of diarrhea.
History & ExaminiationThe dog had shown no signs of improvement after empiric deworming. In addition to diarrhea, the dog had started vomiting the week before presentation. Examination revealed a body condition score of 3/9, elevated body temperature of 103.9°F (normal, 100.5°F–102.5°F), and generalized lymphadenopathy.
DiagnosticsAbdominal ultrasonography revealed hepatomegaly and splenomegaly with peritoneal effusion. CBC and serum biochemistry findings indicated mild normocytic, normochromic anemia with moderate leukocytosis (mature neutrophilia) and hypoproteinemia, along with decreased albumin and globulin levels (Table).In addition, fine-needle aspiration and cytology of the right prescapular lymph node were performed.
Ask YourselfBased on the stained cytology sample shown above:
Is a mixed lymphoid population present, predominated by small lymphocytes?
Are any inflammatory cells identified?
Are other nonlymphoid cells observed?
Diagnosis: Pyogranulomatous lymphadenitis with Histoplasma capsulatum
H. capsulatum, a dimorphic fungal organism, can be readily diagnosed by cytology.1,2 In dogs, weight loss, fever, and inappetence are common clinical findings. Laboratory findings are often nonspecific and can vary, depending on the extent of organism dissemination. H. capsulatum has been identified in the peripheral blood and bone marrow.3 In this case, the patient had disseminated disease and organisms were identified in the spleen, liver, peritoneal fluid, and all peripheral lymph nodes.
Differential DiagnosisThe lymphoid population was mixed and was predominated by small lymphocytes with fewer large and intermediate-size lymphocytes (Figure 1). Many plasma cells were also identified, along with many inflammatory cells, primarily neutrophils and macrophages (Figures 2 and 3). This combination is consistent with pyogranulomatous inflammation.

Figure 1. Small lymphocytes with Histoplasma organisms (arrows) and a mixed inflammatory population, including neutrophils and macrophages, are evident. Scattered fungal yeast organisms can also be seen. (Wright’s-Giemsa stain; original magnification, 500×)

_Figure 2. Yeast organisms are small and round with a crescent-shaped nucleus. Histoplasma organisms (arrows) can also be seen. (Wright’s-Giemsa stain; original magnification, 1000×)
_

Figure 3. Inflammatory cells are present, including neutrophils and macrophages. An extracellular Histoplasma organism (arrow) is also evident. (Wright’s-Giemsa stain; original magnification, 1000×)
Lymphoma can be eliminated as a differential for lymphadenomegaly because of the mixed lymphoid population. Canine lymphoma is more commonly a pure population of large, intermediate, or (less likely) small lymphocytes. Lymphoma consisting of large cells is often the easiest to diagnose because the cytology stain appearance is different from that of a normal or reactive lymph node. Thorough evaluation of the lymphoid population is critical to appropriate interpretation of the node.
This node is likely also reactive. Plasma cells were identified frequently within this sample. Lymph nodes responding to antigenic stimulation commonly have a mixed lymphoid population predominated by small lymphocytes with increased intermediate and large lymphocytes and many plasma cells.
Tumor-draining lymph nodes (ie, nodes immediately downstream of a tumor) may show evidence of metastatic disease. These nodes are often evaluated for the presence of cells that do not belong in a lymph node (eg, epithelial cells) or that may only be observed in the node in low numbers (eg, mast cells). No such cells were observed in this sample.
Inflammatory ProcessesOften, inflammatory processes within the lymph node are subtle. Inflammatory cells within a normal or reactive lymph node can represent a small component of the overall nucleated cell population (commonly <1%). Therefore, subtle increases in inflammatory cells (>5% neutrophils, >3% eosinophils) are significant.4
The inflammatory process is classified by the inflammatory cells observed. If neutrophils are present with few or no macrophages, the process is classified as suppurative lymphadenitis. If eosinophils are present in increased numbers, the process can be classified as eosinophilic lymphadenitis.
The inflammatory population in this case was more obvious, with neutrophils and macrophages outnumbering lymphocytes (Figure 1). The combination of neutrophils, epithelioid macrophages (macrophages seen in pyogranulomatous and granulomatous inflammation that resemble epithelial cells), and occasional multinucleated giant cells (not shown) was consistent with pyogranulomatous lymphadenitis. Yeast organisms are also present in high numbers both free in the background and within the cytoplasm of neutrophils and macrophages (Figures 2 and 3). The organisms are small and round with a thin halo caused by shrinkage of the organism and have eccentrically placed, round to crescent-shaped basophilic centers.1
Did You AnswerBased on the opening stained cytology sample:
Yes, there is a mixed lymphoid population.
High numbers of inflammatory cells, primarily neutrophils with lesser numbers of macrophages, are evident.
The only nonlymphoid cells observed are inflammatory cells, including neutrophils and macrophages. No evidence of metastatic neoplasia could be identified.