Intestinal Dysbiosis
Anna Lena Ziese, MedVet, Clinic of Small Animal Medicine, Centre for Clinical Veterinary Medicine, Ludwig, Maximilian University of Munich
Jan S. Suchodolski, MedVet, DrVetMed, PhD, AGAF, DACVM (Immunology), Gastrointestinal Laboratory at Texas A&M University
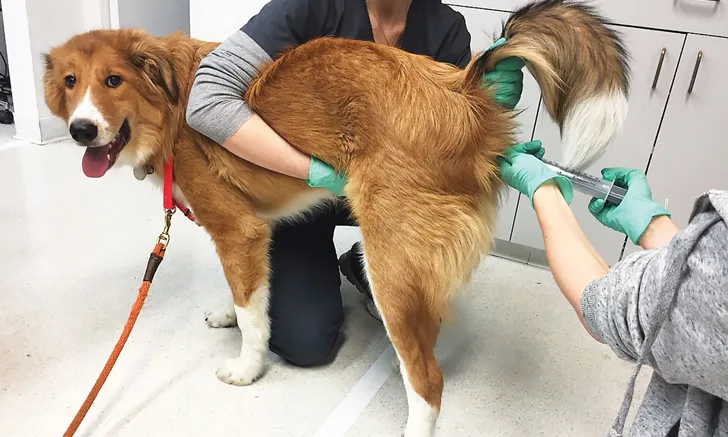
Dog receiving fecal microbiota transplantation via enema
The intestinal microbiome is the genome of all microbes that inhabit the GI tract (ie, bacteria, viruses, fungi, protozoa, archaea). Bacteria are the most abundant group, and every healthy animal shows individual differences in bacterial composition.
Firmicutes, Bacteroidetes, Proteobacteria, and Fusobacteria are the most abundant bacterial phyla, comprising up to 99% of the intestinal microbiota in dogs and cats.<sup1, 2sup> The intestinal microbiome is an important metabolic organ crucial for host health, as it contributes to a variety of physiologic processes throughout the body, including modulating the immune system,3,4 protecting from pathogens, and metabolizing dietary components to provide nutrients (eg, vitamins, short-chain fatty acids).5,6
Background & Pathophysiology
Dysbiosis is defined as alterations in microbial composition and/or diversity. Studies have shown that dogs with inflammatory bowel disease have increased numbers of Proteobacteria (eg, E coli) and decreased numbers of beneficial bacteria such as Fusobacteria, Bacteroidetes, and members of Firmicutes (ie, Faecalibacterium spp, Ruminococcaceae, Turicibacter spp, Blautia spp).7-9 Dogs with acute diarrhea have increased numbers of Clostridium perfringens and decreased numbers of Actinobacter spp, Ruminococcaceae, and Blautia spp.7-9 A key feature of intestinal dysbiosis is not only a decrease or increase of specific bacteria but also a decrease of microbiota function. In some patients, dysbiosis may be the cause of diarrhea, whereas in others, it may be the consequence of underlying intestinal disease. For example, dysbiosis occurs in most patients with GI disease, either along the entire GI tract or more localized to the small or large intestine. The extent of clinical signs varies between patients. Intestinal dysbiosis has also been associated with systemic diseases (eg, diabetes mellitus, obesity) in humans, and similar pathways are currently being investigated in veterinary medicine.10,11
Bile Acid Metabolism as an Emerging Example of Microbiota Dysfunction
Primary bile acids (ie, cholic acid, chenodeoxycholic acid) are produced in the liver, secreted into the intestine, and mostly reabsorbed in the ileum. The intestinal microbiome plays an important role in bile acid metabolism, as intestinal microbiota convert primary bile acids that reach the colon to secondary bile acids (ie, lithocholic acid, deoxycholic acid, ursodeoxycholic acid); therefore, secondary bile acids physiologically occur at a higher concentration in the colon than do primary bile acids.12 Secondary bile acids are important regulators of host homeostasis through activation of various receptors.13 Dysbiosis can result in the dysbiotic microbiota’s inability to convert primary bile acids to secondary bile acids,14 leading to increased primary bile acid concentration in the colon, which may cause secretory diarrhea in humans.15 Anecdotal evidence has suggested that patients with chronic recurrent diarrhea nonresponsive to standard therapy have demonstrated improvements in clinical signs following treatment with bile acid sequestrants that bind bile acids in the colon (eg, cholestyramine).
Diagnosis
Diagnosis of intestinal dysbiosis is similar in dogs and cats. Because most intestinal microbes are strict anaerobes, they cannot be detected via standard bacterial culture methods. Bacterial culture is only useful in detecting specific enteropathogens (eg, Salmonella spp, Campylobacter jejuni) and allows testing for antibiotic susceptibility of these organisms. Culture-independent PCR amplification of bacterial 16S ribosomal RNA genes is the most appropriate tool for assessing the intestinal microbiome,16 but these laboratory methods are usually associated with high costs, require bioinformatic tools, and have a long turnaround time for results. An alternative diagnostic method for dogs is the dysbiosis index, a new quantitative PCR-based diagnostic approach. The dysbiosis index can quantify intestinal dysbiosis by measuring the abundance of 7 bacterial taxa that are known to be altered in GI disease (see Suggested Reading).17
Measurement of serum concentrations of cobalamin and folate to detect potential dysbiosis in the small intestine is currently the most commonly used diagnostic test in dogs and cats.18 In animals with small intestinal dysbiosis, serum cobalamin concentrations may be decreased and/or serum folate concentrations may be increased. Low serum cobalamin concentrations and high serum folate concentrations are highly suggestive of small intestinal dysbiosis, and, in addition to treatment of the underlying disease, supplementation of cobalamin is recommended based on scientific data (see Suggested Reading).
Treatment
Because intestinal dysbiosis plays a role in the pathophysiology of various diseases, normalization of the microbiome—and therefore restoration of bacterial function—is important. The intestinal microbiome can be modulated by prebiotics, probiotics, synbiotics, antibiotics, and/or fecal microbiota transplantation (FMT). Patient differences in microbial composition may result in individualized responses to different therapeutic approaches.
Prebiotics, Probiotics, & Synbiotics
Prebiotics are substrates that are selectively used by host microorganisms and may confer a health benefit to the host.19 They are nondigestible, fermentable or nonfermentable, mainly plant-derived fibers (eg, inulin, nonstarch polysaccharides, disaccharides, oligosaccharides, polysaccharides). Prebiotics are fermented by colonic microbiota to metabolites (eg, short-chain fatty acids),20 which maintain health through a number of different mechanisms (eg, having anti-inflammatory properties, lowering gut pH to prevent growth of pathogenic microbes).21-23 Soluble dietary fibers (eg, psyllium, cellulose, pectin) have water-binding properties to improve fecal consistency, increase thickness of intestinal mucus layers, and have beneficial effects on epithelial cell proliferation. Psyllium has also been shown to have bile acid-binding properties24 and may provide beneficial effects in patients with secretory diarrhea due to bile acid malabsorption. Most commercially available GI diets contain high amounts of fiber, and several studies have shown improved fecal scores following fiber administration.25,26
Probiotics are orally administered live micro-organisms, which, when administered in adequate amounts, provide health benefits to the host.27 Probiotics do not induce major changes in resident microbiota but do have strain-specific mechanisms of action (eg, inhibiting pathogenic invasion,28,29 modulating host immune system, enhancing intestinal barrier function).30 A probiotic should be chosen based on proven effectiveness in scientific studies. Some commercially available probiotics have shown positive therapeutic effects for different GI diseases in animals. For example, studies have reported upregulated intestinal tight junction proteins in the epithelial barrier in dogs with inflammatory bowel disease,30 firmer stool character in shelter cats with uncomplicated diarrhea,31 and decreased fecal abundance of enterotoxigenic Clostridium perfringens and accelerated normalization of the intestinal microbiome in dogs with acute hemorrhagic diarrhea.32
Synbiotics are products with both pre- and probiotics combined and can have synergistic health benefits.33
Antibiotics
Presence of dysbiosis does not equate to an immediate need for antibiotics, as many patients (eg, dogs with food-responsive diarrhea) may also have secondary dysbiosis due to underlying GI pathology. For example, in a study of 136 dogs with chronic enteropathy, only a small subgroup of dogs (11%) had antibiotic-responsive diarrhea,34 which is defined as clinical improvement following antibiotic treatment (eg, tylosin [10-20 mg/kg PO q12h], metronidazole [10 mg/kg PO q12h]). A stepwise therapeutic approach is recommended, particularly in dogs with chronic enteropathy, and antibiotic treatment should only be started if other therapeutic approaches have failed. A dietary trial with additional modulation of the intestinal microbiota via pre- and/or probiotics should be performed before antibiotic treatment is considered, as approximately 60% to 70% of patients with chronic enteropathy respond to hydrolyzed protein or elimination diets alone.34 Although GI signs may resolve within a few days of antibiotic treatment, severe long-term changes in microbiota composition and diversity can result.35,36 The long-term consequences of antibiotic-associated dysbiosis have not been thoroughly investigated, but it is suspected to be associated with atopic, inflammatory, and autoimmune diseases in humans, particularly when antibiotics were administered to the patient early in life.37 In addition, inappropriate antibiotic use promotes development of antimicrobial resistance; thus, antibiotic treatment should be restricted to only specific GI diseases (see Indications for Antibiotic Treatment in Dogs with GI Disease).
INDICATIONS FOR ANTIBIOTIC TREATMENT IN DOGS WITH GI DISEASE
Antibiotic treatment may be indicated in dogs with GI disease, including dogs with:
Chronic enteropathy in which modulation of the intestinal microbiome and dietary trials have failed and with an inadequate response to immunomodulatory/immunosuppressive treatment
Granulomatous colitis with enteroinvasive E coli (eg, boxer colitis)
Neutrophilic intestinal inflammation
Signs of systemic inflammation with acute and/or chronic diarrhea
A high risk for bacterial translocation due to destruction of the intestinal barrier in combination with immunosuppression (eg, dog with hemorrhagic diarrhea and neutropenia due to parvoviral enteritis)
Fecal Microbiota Transplantation
In FMT, stool from a healthy donor is transplanted into the intestinal tract of a patient to achieve normalization of the intestinal microbiome and amelioration of clinical signs (Figure 1). The donor should be healthy, with no history of GI disease; have a normal BCS and fecal score; have a normal intestinal microbiota composition; be free of antibiotic exposure; and, ideally, be fed a hypoallergenic diet. Before FMT, the donor feces should be screened for parasites, enteropathogens, and normal microbiota and bile acid metabolism; donor screening should be repeated approximately every 6 months for as long as the dog serves as a donor.

Dysbiosis index of the fecal microbiota in 3 dogs with chronic inflammatory enteropathy before and 1, 2, 3, and 4 weeks after receiving FMT via nasoduodenal tube. The higher the index (>0), the more dysbiotic the fecal microbiome will be. A negative dysbiosis index (<0) indicates normobiosis. In patients with confirmed GI inflammation, FMT can help improve stool quality but often for only a few days to weeks. Dysbiosis usually returns, followed by recurrence of clinical signs, suggesting that the return of dysbiosis is due to the underlying GI pathology. In patients with chronic inflammatory enteropathy, repeating FMT every 2 to 4 weeks may be useful. FMT can be performed via enema44 or during gastroduodenoscopy.40,41
In human medicine, FMT is an established and effective therapy for recurrent C difficile infections. FMT has also been used to treat inflammatory bowel disease in humans, although with lower success rates.38,39 The success rate of FMT appears to depend on the underlying GI disease, as FMT does not address the underlying GI disease process but rather the associated dysbiosis. In veterinary medicine, little data on FMT exist. Small-scale studies in dogs with chronic inflammatory enteropathy have shown improvement of clinical signs following FMT in a subset of animals, but clinical signs can return within days to months, depending on the individual animal.40,41 Because concurrent GI pathology in those patients was likely the main cause of disease, FMT may serve as adjunct therapy. In the authors’ experience, patients with dysbiosis due to severe GI pathology have not always responded consistently to FMT, and the relapse rate in these patients appears to be higher than in patients with no or mild GI pathology and dysbiosis (Figure 2). Thus, repeated FMTs are recommended in patients with chronic enteropathy. The success rate of FMT is usually higher in patients with acute GI disease42; for example, in puppies with parvoviral enteritis, a faster clinical recovery, reduced mortality rate, and shortened hospitalization time were observed after FMT by rectal enema.43
FMT = fecal microbiota transplantation