Infertility in a Dog
A 9-year-old male Labrador retriever presents with a history of possible infertility.
History. The dog is actively being campaigned at field trials. Over the past 7 years, he has been bred to over 50 bitches with conception rates on average over 80% and average litter size of 6 or more puppies. Early last year, the owner noted some decrease in litter size; in the past few months, there have been few pregnancies and the matings that have resulted in pregnancy have been singleton litters.
Physical Examination. The dog was bright, alert, responsive, and in good body condition. Temperature, pulse, respiratory rate, and mucous membrane examination were normal. Auscultation of the thorax and abdominal palpation were within normal limits. There was no lymphadenopathy.
Digital examination of the prostate per rectum revealed a small, smooth, symmetrical gland. Palpation of the scrotum revealed no abnormalities of the scrotal skin. The testicles were less turgid than normal but symmetrical in size and shape. There were no palpable masses. The epididymides and spermatic cords were within normal limits of size, shape, and texture.
Imaging. Ultrasonographic measurement of the testes revealed total scrotal width to be 3.5 cm. There were no masses noted on ultrasonography, but there were multiple pinpoint hyperechoic lesions throughout the testicular parenchyma of both testes.
Laboratory Analysis. Serum biochemistry (including TT4 and fT4-ED), complete blood count, and urinalysis were within normal limits. Rare sperm were noted on sediment examination of the urine. Semen collection was performed using manual stimulation in the presence of an estrous teaser bitch. The results of semen evaluation are shown in the Table.
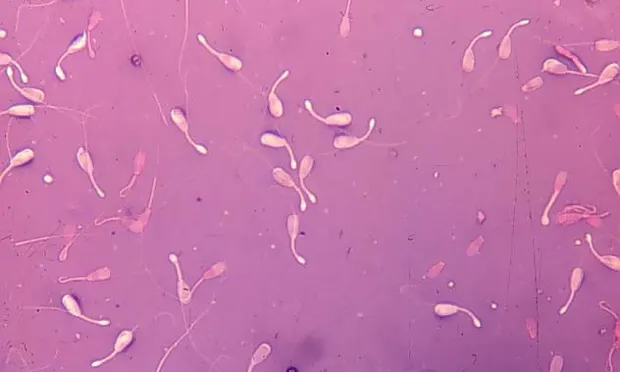
A sample of the ejaculate was stained with eosin-nigrosin and allowed to air dry (Figures 1 through 3). Another sample of the ejaculate was stained with modified Wright's-Giemsa to evaluate round cells (Figure 4).
ASK YOURSELF ...
What are the primary defects noted in Figures 1 through 3?
What is the round cell in Figure 4?
If these sperm are representative of the ejaculate, what is the morphologic assessment of the semen sample?
Diagnosis: Primary testicular degeneration
Cytologic Assessment. Morphologic characteristics must be evaluated on fixed, stained semen samples (see Box) under oil immersion (100×), counting a minimum of 100 cells. Use of low to medium (10× to 40×) power wet mounts to assess sperm morphology often results in many missed or misclassified morphologic defects. Use of formol-buffered saline to fix the spermatozoa and subsequent assessment under high power, using phase contrast or differential interference contrast microscopy, is also acceptable but may be unavailable to the general practitioner.
Assessment of the sample should define defects as primary (occurring in the testicle during spermatogenesis) or secondary (occurring during epididymal storage, ejaculation, or semen handling). More than 70% normal sperm is considered acceptable. Morphologic assessment in this case (Figures 1-3) revealed 15% normal sperm, 34% distal midpiece reflexes, 5% bent midpieces, 16% detached heads, 20% pyriform heads, and 10% knobbed acrosomes. Two large, binucleate germ cells are seen on the slide stained with Wright's-Giemsa (Figure 4).
Discussion. This sample is typical of a dog with primary testicular degeneration. An increase in primary defects is common -- specifically, increased numbers of defects of the sperm head and acrosome, primary defects of the midpiece, and high numbers of detached heads (indicative of abnormalities in midpiece attachment to the head) are noted with primary testicular disease and testicular degeneration. In some cases there may be small to large round cells (germ cells or white blood cells) in the sample. The number of germ cells increases as testicular degeneration progresses.
Potential causes of primary testicular degeneration include senescent change, autoimmune orchitis and epididymitis, drugs (anabolic or sex steroids, long-term use of nonsteroidal antiinflammatory drugs or glucocorticoids, environmental toxins), chronic endogenous glucocorticoid release (stress), chronic orchitis/epididymitis or prostatitis, _Brucella canis i_nfection, neoplasia, scrotal overheating, or pyrexia associated with systemic illness.
Further Diagnostics. Fine-needle aspiration or testicular biopsy may provide a definitive diagnosis, and in some cases, the cause of the disorder. Fine-needle biopsies typically have low cell density and spermatogenic cells of varying stages of maturation (large round cells to mature spermatozoa). Sertoli cells are readily aspirated while Leydig cells are not.
Assessment of thyroid function, specifically autoantibodies to T 3, T4, o thyroglobulin, may be helpful, as there has been a hereditary association between autoimmune thyroid disease and autoimmune orchitis and epididymitis. The semen should be evaluated again in 60 days to determine if the abnormalities are permanent or transient.
DID YOU ANSWER ...
Distal midpiece reflexes, bent midpieces, detached heads, pyriform heads, knobbed acrosomes
Germ cell
Teratozoospermia, asthenozoospermia, and oligozoospermia