Infectious Respiratory Disease in a Dog
Joanna K. Fry, DVM, Gulf Coast Veterinary Specialists
Derek P. Burney, DVM, PhD, DACVIM, Veterinary Specialists of North Texas
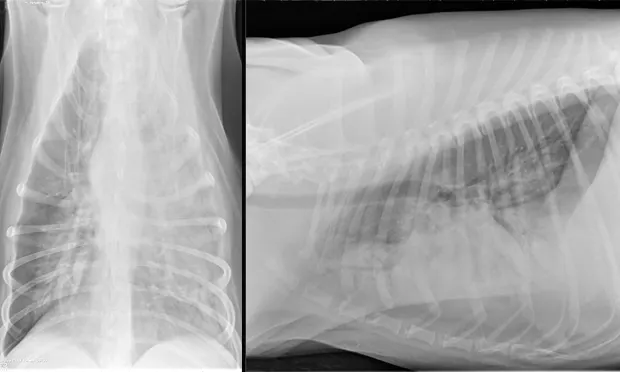
An alveolar pattern in the entire left hemithorax and in the hilar and midzone regions of the right caudal lung lobe. Multiple air bronchograms are observed in the left hemithorax on both views.
A 4-year-old castrated goldendoodle was referred with progressive cough of approximately 1-week duration.
History
The patient had been receiving antibiotics and corticosteroids for suspected pyoderma. Thoracic radiographs revealed an alveolar pattern in the left cranial and caudal lung lobes, consistent with pneumonia. The patient was hospitalized for supportive care and received IV fluids, cough suppressant, and antibiotic therapy (ie, enrofloxacin, doxycycline). The patient became febrile and hypoxemic and was referred for further evaluation and supportive care.
Examination
The patient was quiet, alert, and responsive, had a rectal temperature of 103.7°F, was panting heavily, and demonstrated increased bronchovesicular sounds bilaterally. A moist productive cough was noted. Pulse oximetry was 91% on room air (range, 98%–100%). See Table: Key Laboratory Findings for results of blood chemistry panel.
Treatment
The patient was hospitalized and placed on Plasma-Lyte at 90 mL/kg IV q24h (abbottanimalhealth.com), enrofloxacin at 5 mg/kg IV q24h, ampicillin and sulbactam at 40 mg/kg IV q8h, and clindamycin at 10 mg/kg IV q12h. Nebulization with amikacin and chest coupage was performed q6h.
Oxygen therapy was administered via a single nasal oxygen catheter, but hypoxemia persisted until placement of a second nasal oxygen catheter. Despite implementing these protocols, the patient’s respiratory effort and rate remained increased.
Recheck thoracic radiographs obtained 35 hours after admission revealed a progressive alveolar pattern affecting the left cranial, left caudal, and right caudal lung lobes. Consolidation was noted in the left cranial lung lobe (Figure 1, above).
Table: Key Laboratory Findings
Ask Yourself...
Discussion
Diagnostic Evaluation
Diagnostic evaluation of pulmonary parenchyma is invasive, is associated with varying degrees of morbidity and mortality, and often requires general anesthesia. Patients with severe respiratory compromise are often oxygen dependent and are not ideal anesthetic candidates. Noninvasive screening tests are often recommended before proceeding with more invasive diagnostics, depending on patient status, duration of clinical signs, and response to previous therapies.
Initial diagnostic plan includes CBC, biochemistry profile, and urinalysis. Hematogenous spread of bacterial pneumonia also warrants urine culture. Baermann fecal analysis evaluates common intestinal parasites and lungworms, while zinc sulfate centrifugation fecal flotation evaluates for the presence of flukes. Because of intermittent organism shedding, a negative result does not rule out parasitic disease, and empiric treatment with fenbendazole may be warranted.
Respiratory disease panels that utilize real-time polymerase chain reactions (RT-PCRs) can be used to detect bacterial and viral organisms often implicated in infectious tracheobronchitis disease complex, including Bordetella bronchiseptica, canine parainfluenza virus, canine adenovirus type 2, canine distemper virus, canine respiratory coronavirus, canine herpesvirus, and canine influenza virus.
Fungal serology to evaluate antibody titers or urine antigen screening assays may help rule out fungal disease in endemic areas.
Further evaluation of pulmonary parenchyma can help obtain a more definitive diagnosis. Transtracheal or endotracheal lavages are considered minimally invasive and relatively safe and can be performed earlier in the disease process if the owner is willing. Samples are collected from the large airways, so these diagnostic techniques should only be employed to diagnose pulmonary parenchyma disease when cough is productive.
Bronchoalveolar lavage follows the same technique as transtracheal or endotracheal lavage, but the sample is collected from the lower airways and does not rely on a productive cough (Figure 2). When pulmonary parenchymal disease is isolated to 1 region, bronchoscopy can help guide sample collection. Fine-needle aspiration of the pulmonary parenchyma is considered invasive and may be associated with iatrogenic pneumothorax. Although it is not considered the test of choice for diffusing disease, sensitivity is high with focal lesions.

Fluid from a bronchoalveolar lavage. Inflammatory cells predominate, consisting of approximately 70%–80% nondegenerate neutrophils, followed by approximately 20%–30% vacuolated macrophages within a granular fluid background. (50× original magnification) Courtesy IDEXX Laboratories
Lung biopsies are warranted when patients fail aggressive therapy and previous diagnostics have been unrewarding. However, they are considerably more invasive and may be associated with greater morbidity. Multiple techniques are available when considering a lung biopsy, including thoracoscopy, thoracotomy, or a key-hole approach.
Differentials & Prognosis
Conditions that affect the pulmonary parenchyma and may result in an alveolar pattern include infectious pneumonia (bacterial, fungal, protozoal, viral, parasitic), aspiration pneumonitis, aspiration pneumonia, cardiogenic and noncardiogenic pulmonary edema, hemorrhage, pulmonary thromboembolism, neoplasia, acute lung injury, and acute respiratory distress syndrome.
The most common cause for infectious respiratory disease is bacterial pneumonia, usually with Escherichia coli, Pasteurella spp, Bordetella bronchiseptica, Streptococcus spp, coagulase positive Staphylococcus spp, Klebsiella pneumoniae, Enterococcus spp, and Pseudomonas aeruginosa. Fungal infections are common in certain regions throughout the United States and include infections with Blastomyces spp, Histoplasma spp, Cryptococcus spp, Coccidioides spp, and Aspergillus spp (Figure 3). Protozoal causes for pneumonia are uncommon, but Toxoplasma gondii should be considered. Common intestinal parasitic infectious organisms include Toxocara spp, Ancylostoma spp, and Paragonimus kellicotti, a lung fluke common in the Great Lakes, midwest, and southern regions of the United States. Although uncommon, rickettsial pneumonitis has been implicated in cases of Ehrlichia canis and Rickettsia rickettsii infection.

Fluid from a bronchoalveolar lavage. Note large numbers of inflammatory cells and many aggregates and individualized, uniform, columnar, ciliated, and nonciliated respiratory epithelial cells. Inflammation consists of large numbers of nondegenerate and mildly degenerate neutrophils and foamy alveolar macrophages. Extracellular and phagocytized yeast, small, spherical, measuring approximately 1–3 microns in diameter, are present (arrows). The yeast have a slight clear capsule and a basophilic internal stippled nucleus, consistent morphologically with Histoplasma spp. (100× original magnification) Courtesy IDEXX Laboratories
Prognosis for patients with infectious respiratory disease is variable and depends on the underlying cause, response to treatment, and presence of concurrent disease. Supportive care including oxygen supplementation, fluid therapy to prevent dehydration, and nutritional supplementation may be required. Cough suppression, although controversial and thought to be inappropriate in infectious disease cases, may provide some relief.
Diagnosis & Treatment
Given the progressive changes on the thoracic radiographs and overall lack of therapeutic response, a fine-needle aspiration of the lungs was performed. This analysis was nondiagnostic. A transtracheal wash was performed and cytologic evaluation revealed primarily degenerate neutrophils with both intracellular and extracellular rods. The bacteria occasionally occurred in long chains. Cytologic evaluation was consistent with septic suppurative inflammation.
Nebulization with acetylcysteine and aminophylline (10 mg/kg IV q8h) was added to the treatment regimen. A persistent fever 48 hours after admission led to addition of acetaminophen at 10 mg/kg IV q12h. While the fever improved, the patient’s temperature did not return to normal. Carprofen at 2 mg/kg PO q12h was initiated and the temperature normalized.
The aerobic culture yielded growth of 2 organisms: abundant growth of Klebsiella pneumoniae and light growth of Corynebacterium spp. Anaerobic culture was negative; based on aerobic culture results, clindamycin therapy was discontinued and doxycycline was initiated at 5 mg/kg PO q12h.
Outcome & Follow-up
The patient recovered well and was discharged 7 days after admission. Outpatient therapy was continued with enrofloxacin at 10 mg/kg PO q24h, doxycycline at 5 mg/kg PO q12h, and amoxicillin trihydrate–clavulanate potassium (Clavamox, animalhealth.pfizer.com) at 13.75 mg/kg PO q12h 2 weeks after radiographic resolution of pneumonia (Figure 4) for a total of 7 weeks.
The patient made a full recovery.
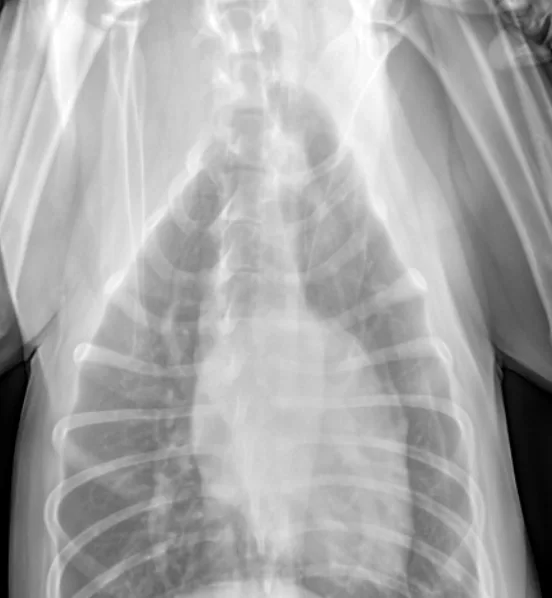
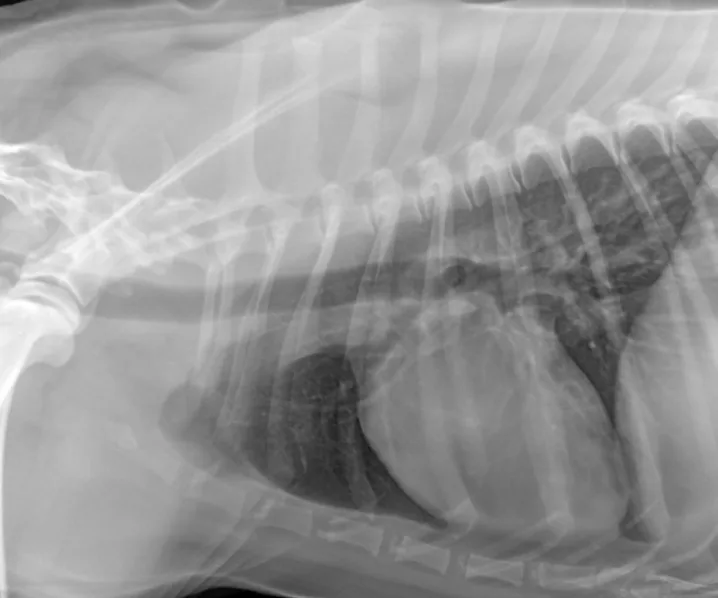
In follow-up films, alveolar infiltrates are completely resolved. There is a very slight increased nonstructured interstitial pattern within the left caudal lung lobe, which could be artifact as a result of mild to moderate leftward obliquity or possibly pulmonary fibrosis secondary to previous pneumonia. Courtesy Montrose Veterinary Clinic
The Take-Home
While the most common cause for infectious respiratory disease in dogs is bacterial pneumonia, other infectious agents should be considered.
Treatment using antimicrobial therapy should be based on organism identification and culture and susceptibility testing.
Evaluation of the pulmonary parenchyma carries additional risks. Patient status, response to treatment, and risks involving the diagnostic evaluation should be considered.
Antimicrobial therapy should be continued past resolution of clinical and radiographic signs to help prevent relapse.