Dermatophytosis
Karen A. Moriello, DVM, DACVD, University of Wisconsin–Madison
Dermatophytosis (ie, ringworm) is a superficial fungal disease of the skin, hair, and claws that affects 1% to 4% of cats and dogs worldwide.1
Background & Pathophysiology
The most common dermatophyte pathogens in small animals are Microsporum canis, M gypseum, and Trichophyton spp.1 M gypseum is found in and contracted from soil; Trichophyton spp are presumably transmitted through contact with large animals or rodents.1
Dermatophytosis is more common in warm, humid climates and is seasonal in temperate climates.1 The primary risk factor is exposure to another infected animal. Group housing of animals (eg, pet shops, animal shelters, animal rescue situations, hoarding) can also increase risk for disease.1
Successful development of an infection nidus requires exposure of the skin surface to a critical load of infective spores (ie, arthrospores), increased moisture on the skin surface to facilitate sporulation, and microtrauma. The latter is believed to be important in facilitating infection, as experimental infections in cats have been difficult to establish without it.1 Under optimal conditions, infective spores can adhere to skin within 6 hours, germinate within 24 hours, and begin shedding within 7 days.1 Lesion foci are detectable within 7 days; however, pet owners may not notice lesions until 14 to 21 days postexposure.1
The primary mode of transmission is via direct contact with an infected animal. Transmission can also be from contaminated fomites, especially those that can cause microtrauma (eg, clipper blades, grooming tools, collars).
Transmission via a contaminated environment has been shown to be inefficient in establishing active lesions.1 In the author’s experience, exposure to a contaminated environment most commonly results in culture-positive, lesion-free fomite carriage; however, this exposure can be a risk factor for transmission if the patient is debilitated, has chronic skin issues, and/or has any inflammatory skin disease.
History
Patients of all ages and breeds can be affected, but young animals and those under severe physiologic stress are predisposed.1 Persian cats, Yorkshire terriers, and Jack Russell terriers appear to be overrepresented; subcutaneous nodular lesions have been observed almost exclusively in Persian cats and Yorkshire terrier dogs.1 Working and hunting dogs are predisposed to kerion lesions, which are focal areas of dermatophytosis that resemble nodular draining lesions of deep pyoderma.1
Clinical history of dermatophytosis is variable. A history of skin lesions in a patient recently adopted from a high-risk situation should raise suspicion. Owners may also report skin lesions on other animals in the home and/or on themselves.
Related Article: Image Gallery: Immune-Mediated Skin Diseases
Related Article: Local Anesthetic Blocks of the Distal Limbs for Dermatologic Procedures
Clinical Signs
Hair loss, scaling, crusting, and erythema are the most common clinical lesions. Lesions tend to be asymmetric and can affect any area on the body but often appear first on the face, ears, and distal extremities. Lesions may be difficult to find in longhaired animals. Facial lesions appearing as pustular dermatophytosis (ie, resembling pemphigus foliaceus) are rare. Lesions may be focal, multifocal, or widespread, and disease may be mild to severe; presentation reflects the health of the host.
Pruritus may be present and is highly variable; some lesions may be intensely pruritic and exudative and may resemble superficial pyoderma in dogs or exudative eosinophilic lesions in cats.
Nodular inflammatory lesions may be observed in dogs or cats, especially working dogs; nodular lesions in cats may be exudative or subcutaneous.
Purulent paronychia may be observed. Concurrent bacterial infection may worsen clinical signs.
Diagnosis
Although dermatophytosis is a differential diagnosis for any inflammatory follicular disease, it is more commonly diagnosed in kittens and puppies and less frequently diagnosed in adult animals. In adult dogs, superficial pyoderma, demodicosis, and other ectoparasitic infestations that can cause hair loss, erythema, and/or scaling should be ruled out. In adult cats, flea infestation, flea allergy dermatitis, and other infestations and allergies associated with generalized scaling should be ruled out.
Dermatophytosis and pemphigus foliaceus can have similar presentations in adult animals and can be differentiated by histopathology, cytology, direct examination of hairs, and fungal culture.
No gold-standard diagnostic test is available for dermatophytosis.1 Histopathology (± tissue culture) can confirm nodular or pustular dermatophytosis, but fungal culture must be performed to determine fungal species (see Point-of-Care Diagnostics). In a study, direct microscopic examination of hairs (ie, trichogram) and skin scrapings from lesions confirmed infection in >85% of cases.1 Fungal culture can be used to identify spores on the hair coat and confirm disease if the sample is from a lesion site. Positive fungal cultures from whole-body toothbrush samples may be due to true disease or fomite carriage; thus, sampling should be limited to lesion sites. Dermatophyte PCR is sensitive and specific for fungal DNA but detects both viable and nonviable fungal DNA.1 PCR has a quicker turnaround time than does fungal culture, but laboratory access may be limited. Field studies comparing fungal culture and PCR are few, so it is not possible to comment on how concordant results are between the tests. If PCR is pursued, a large number of hairs and crusts only from the target lesions should be submitted to the laboratory. A toothbrush fungal culture should also be sent to the laboratory in case it is needed to confirm the infection. The author’s first choice is a fungal culture.
Point-of-Care Diagnostics
Microscopic Examination of Hairs
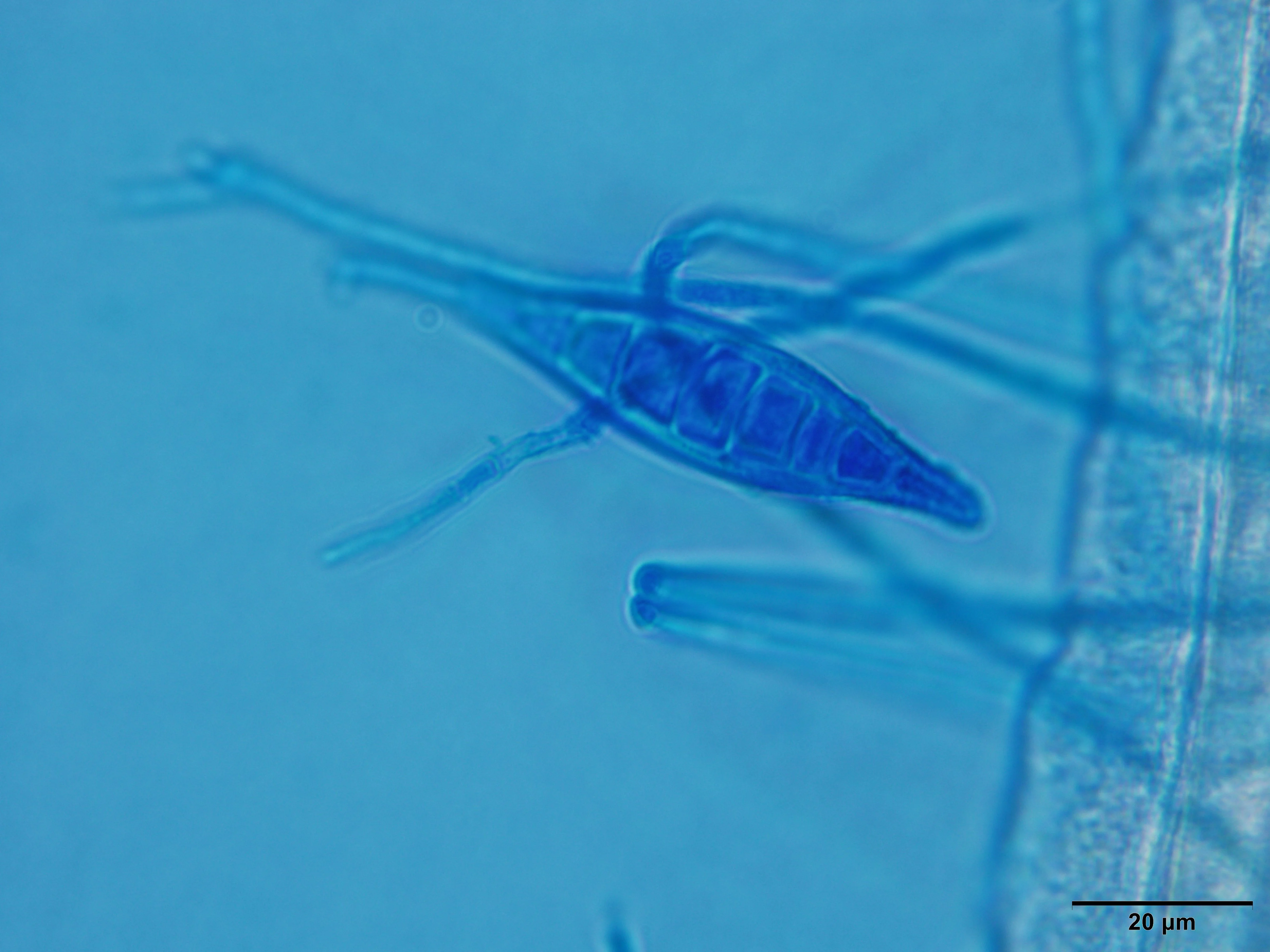
Hairs should be plucked in the direction of growth and skin scrapings obtained from the lesion margins. Mineral oil should be used for mounting. Clearing agents used to soften nail keratin in human medicine (eg, potassium hydroxide) are not needed and can add artifacts or damage microscope lenses, as they are caustic. Cover slips should be placed on slide specimens and viewed at 4× and 10× magnification. Infected hairs are wider and paler in appearance, and ectothrix spores cuffing the hair may be present (Figures 1 and 2).

Microscopic view of an infected hair wider than normal hairs; internal structures of the hair shaft are not visible. 10× magnification. Image courtesy of Dr. Karen A. Moriello

Microscopic view of a newly infected hair wider than surrounding hairs; some internal structures (eg, dark black pigment; arrow) are visible on the proximal part of the hair. 4× magnification. Image courtesy of Dr. Karen A. Moriello
Wood’s lamp examination is recommended when dermatophytosis is suspected. This is not a diagnostic test but rather a diagnostic tool that helps find suspect M canis-infected hairs for direct examination. In recent studies, 91% to 100% of patients with untreated spontaneous infections showed positive fluorescence.1 Historic studies reporting 30% to 50% positive fluorescence were from retrospective laboratory studies and not from in vivo studies.
A plug-in Wood’s lamp with a wavelength of 320 to 400 nm and built-in magnification should be used. During examination, the clinician should hold the lamp 2 to 4 cm from the patient’s skin and proceed slowly, starting at the head. Apple-green fluorescence on the hair shaft is suggestive of M canis infection. Crusts do not glow and may need to be lifted (Figure 3) to find infected hairs underneath.
If infection is confirmed with point-of-care diagnostics, treatment can be initiated.

Wood’s lamp examination of hairs. The classic apple-green fluorescence of M canis, the only important veterinary pathogen that fluoresces, can be noted. Image courtesy of Dr. Karen A. Moriello
Fungal Culture
Fungal culture can be performed via point-of-care or laboratory diagnostics. The most commonly used fungal culture medium is dermatophyte test medium (DTM). If fungal culture is performed in-house, easy-open or petri dish-type plates with the largest surface area possible should be used. Fungal culture jars can be difficult to inoculate, are prone to increased bacterial overgrowth due to increased humidity, and can be difficult to obtain samples from the surface. Removing media from the jars is not recommended, as this increases exposure to a possible pathogen.
An untreated lesion should be sampled using a soft-bristled toothbrush, which is mycologically sterile if prepackaged. It is important to sample the skin surface and hairs; materials will be entrapped in toothbrush bristles. Crusts can be gently lifted to sample beneath them using the edge of a skin scraping spatula or other blunt-edged instrument.
In-house fungal cultures can be performed on site, and one study showed that the difference in results between point-of-care testing and a reference laboratory was <3% if proper procedures were followed.2 In-house testing practices should follow laboratory biohazard practices.
Fungal cultures should be incubated at room temperature and examined daily; darkness is not necessary. M canis cultures can be finalized at day 14 if no growth is observed.3,4 Studies have not been conducted for M gypseum, but this pathogen is not difficult to isolate, and it is reasonable to extrapolate studies from M canis to this pathogen. Regarding Trichophyton spp, a study in humans examined 5549 samples and found only 16 required >17 days of incubation, and only 1 of 16 was a veterinary pathogen (T mentagrophtyes).3,4 The authors concluded 17 days is adequate for finalizing a diagnosis for human pathogens.4
Microscopic identification must be performed to confirm diagnosis (see Suggested Reading). Pale, flat, and fluffy gross colonies should be sampled for microscopy. If DTM is used, clinicians should look for pale colonies with a red color change in the medium around them as they grow. It is important to remember that the red color change is not diagnostic of a dermatophyte. Colony morphology may also be suggestive of a positive fungal culture, but it is not diagnostic; microscopic identification is always needed.
Related Article: Technical Terms for Dermatologic Lesions
Related Article: Skin Biopsy for Diffuse Dermatologic Disease in Cats & Dogs
Treatment & Management
In otherwise healthy animals, dermatophytosis usually self-resolves; treatment is intended to shorten the course of disease and limit contagion. Owners should be informed that dermatophytosis is a non-life–threatening zoonotic disease that causes easily treatable skin lesions and be instructed to consult their personal physician if they have questions or suspect they may have skin lesions. Misinformation regarding cleaning, disinfection, and environmental contamination is pervasive; owners should be advised that fungal spores do not invade home surfaces as do other molds (eg, mildew), do not cause respiratory disease, and can be easily removed.
Owners should also be informed that treatment is multimodal and includes reasonable confinement, cleaning, topical therapy, systemic therapy, and monitoring.
Confinement
Confinement of patients limits the area that requires cleaning and helps prevent the spread of disease. Confinement alone is not curative and should be implemented with care to ensure the welfare of the patient; the area should be a single room large enough to allow eating, sleeping, and exercise.
Dermatophytosis can occur in young animals during key socialization and bonding times; owners should continue to socialize and play with the infected pet. Owners should wear gloves and washable clothing and avoid direct skin-to-skin contact. Hand hygiene is important; owners should wash hands or use hand sanitizer (found to be sporicidal in the author’s laboratory) if soap and water are not available. Safe, washable, interactive toys should be provided. Recommendations for other animals in the home are similar to those for any infectious disease: Direct contact should be avoided, and bowls, brushes, leashes, and bedding should not be shared among animals. Keeping animals physically separated can be challenging; in-contact animals can be bathed with a topical antifungal shampoo (see Topical Therapy) or treated with lime sulfur and watched closely for development of lesions.
Cleaning
Cleaning removes shed-infective material in the environment. Cleaning minimizes false-positive fungal cultures (ie, a culture-positive but lesion-free patient) that complicate determination of mycologic cure and prolong treatment. If cleaning is regularly performed while the patient receives topical therapy, most homes can be decontaminated with 1 or 2 cleanings after cure.5 Any items that can be mechanically washed can also be decontaminated.1
Homes do not need to be aggressively cleaned every day; twice-weekly thorough cleaning of the confinement area is usually sufficient. It is important to mechanically remove gross debris (ie, hairs) on a daily basis. The most efficient method is vacuuming, provided the vacuum has a filter to trap debris and is emptied and cleaned after each use. Removal of debris with disposable dust cloths or wet wipes is adequate between aggressive cleanings.
After removal of gross debris, hard surfaces should be washed with detergent until visibly clean, then rinsed, dried, and sprayed with a disinfectant. Over-the-counter, ready-to-use bathroom disinfectant cleaners with a label claim that it is an antifungal against Trichophyton spp or products containing accelerated hydrogen peroxide are effective against dermatophytes.6 Bleach should be avoided, as it can be an irritant, can damage surfaces, and has no detergent properties.
Exposed textiles and soft items should be washed twice with any common laundry detergent on the longest wash cycle possible. Bleach and/or hot water have not been found to be superior to cold water without bleach.7 Fabric should be dried according to its label instructions. Agitation from washing (not drying) is antifungal; household dryers do not reach temperatures that are sporicidal. Carpets can be decontaminated by being washed with a beater-brush rug cleaner twice or steam cleaned once. If a disinfectant is desired, antifungal pet shampoo can be substituted for carpet detergent, but color testing should be performed prior to use.8
Pet food bowls can be decontaminated via thorough washing with hot, soapy water.9
Topical Therapy
Topical treatment of the hair coat is not considered an optional part of therapy. Topical therapy decreases shedding of infective material, kills ectothrix spores (not affected by systemic therapy1) on the hair coat, helps prevent development of new lesions, and decreases contagion and environmental contamination. Clipping of the hair coat is not routinely needed, but infected hair may be clipped with metal blunt-tip scissors (ie, to avoid microtrauma to skin from electric clippers). The coat should be combed before application to remove loose hairs. Whole-body hair coat disinfection twice weekly is recommended. Patients should be kept warm (eg, with warm blankets) following whole-body treatment to prevent hypothermia.
In vitro and in vivo studies have shown lime sulfur, miconazole/chlorhexidine gluconate, and enilconazole to be consistently effective.1 Leave-on rinses are preferred because of their residual activity and should be applied to the face with a sponge.
Leave-on lime sulfur rinse should be applied twice weekly at a 1:16 dilution. This product, which is not available in all countries, may discolor the hair coat and is somewhat odorous. It will also stain fabric and discolor items in contact with it; owners must wear gloves when applying the product and should not let it come into contact with watches or jewelry. Owners should be educated about proper dilution, as concentrated application can be irritating to the skin. Lime sulfur is efficacious, is immediately sporicidal, and has residual activity. Lime sulfur can be drying to the hair coat or footpads when used for prolonged periods. In the author’s experience in shelters, oral ulcers were never observed as a result of use of lime sulfur; cats with oral ulcers had concurrent respiratory infections.
Miconazole (2%)/chlorhexidine gluconate (2%) shampoo is widely available and is sporicidal but does not have residual activity. Although no in vivo studies have determined the optimal contact time, an in vitro study found that 3 minutes of contact was sporicidal10; therefore, 3 to 10 minutes of contact is recommended. This shampoo can also be used for treatment of exposed but uninfected animals in the home.
Enilconazole leave-on emulsion (1:50 or 1:100) is only labeled for use in cats in France and is not available in all countries. The emulsion is slightly odorous and may be greasy.
Adjuvant focal topical therapy applied once daily is recommended for focal lesions and/or lesions in areas that are difficult to treat (eg, face, ears). This is in contrast to recommendations in most veterinary dermatology textbooks that recommend application twice daily. A recent in vitro study demonstrated good residual activity of clotrimazole (1%), terbinafine (1%), miconazole (0.2%, 1%, or 2%), and 3 leave-on mousse products containing chlorhexidine and climbazole, miconazole, or ketoconazole.10 Mousse products may be suitable for animals that cannot be wetted or are difficult to treat. Care must be taken to use the product as directed by the manufacturer. For periocular lesions, 2% miconazole nitrate vaginal cream is recommended11,12; this product is widely used with proven safety by ophthalmologists to treat fungal keratitis. Lesions on or in the ears are best treated with otic preparations with antifungal efficacy.
Owners should always wear gloves when applying topical therapy.
Systemic Therapy
Systemic therapy eradicates infection in the hair follicle and is considered to be an important and necessary part of therapy. Itraconazole (noncompounded) and terbinafine are the most effective and safe treatments for dermatophytosis and have residual activity in the skin and hair, allowing for pulse therapy. Compounded itraconazole should not be used due to poor bioavailability.13,14 In cats, itraconazole should be administered at 5 mg/kg PO once daily on a week on/week off basis until mycologic cure (see Guidelines for Determining Mycologic Cure). Because this drug is difficult to get into a suspension, the veterinary or human pediatric liquid suspension should be used. If neither is available, 100-mg capsules can be repackaged into 25-mg capsules.
Experimental and field studies have found this drug to be well tolerated, with the most adverse side effects being vomiting and/or decreased appetite.1 In a licensing study, elevations of liver enzymes posttreatment were noted but deemed to be of little clinical significance, and most remained within normal laboratory values.15 In a shelter study, 21 cats with dermatophytosis had serum chemistry profile results monitored pre- and posttreatment (ie, itraconazole at 5 mg/kg PO once daily for 21 days). No cats became ill or anorexic. A statistically significant increase in alanine aminotransferase was noted, but no values were outside normal reference ranges.16 Based on these findings, routine monitoring of liver enzymes is not routinely needed in otherwise healthy animals. Monitoring serum chemistry profiles is important in animals with comorbidities.
Alternatively, terbinafine (30-40 mg/kg PO once daily) may be administered until mycologic cure. Some cats given terbinafine may experience GI effects. In small dogs, itraconazole at 5 mg/kg PO may be administered once daily. Pulse therapy options (eg, week on/week off) may likely be appropriate for dogs, but this has not been confirmed. In larger dogs, terbinafine at 30-40 mg/kg PO should be administered once daily.
Griseofulvin, which is fungistatic and teratogenic and requires intensive monitoring, is no longer recommended because safer choices exist. Ketoconazole and fluconazole are not recommended because they do not have residual activity in the skin and have higher MIC than do itraconazole and terbinafine.
Monitoring
Infected patients should be treated until mycologic and clinical cure are achieved (see Guidelines for Determining Mycologic Cure). Clinical cure commonly precedes mycologic cure and can occur within weeks of treatment initiation. A recent study showed that when compliance with treatment and environmental cleaning was high, 1 negative fungal culture was predictive of mycologic cure.16 If 2 negative fungal cultures are needed (eg, patient has underlying illness, compliance issues are suspect), they should be obtained at weekly intervals.
Prognosis is good in patients with superficial dermatophytosis and dogs with kerion reactions. Prognosis is less certain in patients with SC nodular lesions; these patients, particularly cats, often require surgical intervention and long-term therapy.
Immunocompromised humans should avoid contact with infected animals during treatment, as is the case in any animal-acquired disease.17
Guidelines for Determining Mycologic Cure
Topical therapy should be continued pending the results of all posttreatment fungal cultures.
In otherwise healthy animals, the first posttreatment fungal culture should be obtained after the prescribed treatment protocol is completed and the patient is lesion free with negative Wood’s lamp results for M canis infections. If the culture is negative, the patient could be assumed cured.
In patients that were clinically ill at the time of diagnosis, were unthrifty (eg, not clinically well), or for which there is concern about treatment compliance, the first posttreatment fungal culture should be delayed until there is both clinical cure and resolution of any underlying medical problems or treatment issues. Two consecutive fungal cultures at weekly intervals are recommended to ensure cure.
Clinical Challenges
Culture-positive, lesion-free cats are typically fomite carriers or have subtle lesions not detected at initial examination and should be carefully re-examined (including the head and between the digits) with a Wood’s lamp. Wood’s lamp examination may identify lesions or sites of early infection not visible under examination room light. If no lesions are found, the cat should be bathed with an antifungal shampoo or treated with a lime sulfur rinse; the fungal culture should be obtained again when the cat is dry. Topical therapy should be continued until fungal culture results are available. Commonly, these cats rapidly become fungal culture-negative when allowed to groom and moved to a clean area.
A persistent positive fungal culture following clinical cure has 3 common causes: inadequate disinfection of the hair coat, fomite carriage due to insufficient cleaning of the home, and development of new lesions in areas that are difficult to treat due to inadequate hair coat disinfection. In the case of fomite carriage, owners should be instructed to aggressively clean the patient’s living area and repeat topical therapy; culture testing should be performed again when the cat is dry or within 24 hours.