Clinician's Forum: Expert Views from a Roundtable on Apoquel

Brought to you by Zoetis
Participants
Cheryl London, DVM, PhD, DACVIM (Oncology), Anne Engen & Dusty Professor of Comparative Oncology, Cummings School of Veterinary Medicine at Tufts University
Douglas DeBoer, DVM, DACVD, Professor of Dermatology, University of Wisconsin–Madison
Lindsay McKay, DVM, DACVD, Veterinary Dermatologist, VCA Arboretum View Animal Hospital, Downers Grove, Illinois
Fiona Bateman, BVSc, MANZCVS, DACVD, Veterinary Dermatologist, Animal Dermatology Clinic, Marietta, Georgia
Peter H. Eeg, DVM, Veterinarian, Poolesville Veterinary Clinic, Poolesville, Maryland
Dan Hebert, DVM, Veterinarian, Duxbury Animal Hospital, Duxbury, Massachussetts
Dawn Cleaver, DVM, Associate Director of Global Regulatory Affairs Zoetis, Kalamazoo, Michigan
Andrea Gonzales, PhD, Senior Research Director, Companion Animal Research Zoetis, Kalamazoo, Michigan
Moderator
Patricia Stark, President, Patricia Stark Communications
Key Takeaways
First-line use of Apoquel can result in rapid relief of allergic pruritus and inflammation, giving owners peace of mind and enhancing their trust in the veterinarian and their recommendations.
More than 6 million treated dogs and 5 years of pharmacovigilance data for Apoquel
There is no published information that would indicate an increased risk for development of neoplasia associated with administration of Apoquel.
Understanding Apoquel & Dispelling the Myths
Apoquel® (oclacitinib tablet) is a revolutionary medication that provides fast, effective, and safe relief of allergic itch, improving the lives of dogs and their owners. This discussion focuses on the attributes of Apoquel and areas of misunderstanding since its launch 5 years ago.
Patricia Stark: Are there currently any published and widely accepted guidelines for the treatment of acute flares of allergic dermatitis?
Dr. DeBoer: There are guidelines that were published initially in 2010 and most recently updated in 2015 that give us the best evidence for treating either acute atopic dermatitis or chronic disease. With acute atopic dermatitis, 3 things are important in treatment. The first is identifying flare factors, such as infections, parasites, and exposure to food allergens to which the dog is sensitive. The second is good general care of the skin and hair coat (ie, bathing to remove bacteria, yeast, and allergens). And last is relief of the pet’s itch. Short-term treatment options that act quickly to relieve itch include Apoquel and corticosteroids.
Patricia Stark: Guidelines are a valuable starting point, but do they miss something that is relevant to treatment success?
Dr. McKay: I think they do. Most owners have a negative perception of steroids, and they really don’t want to use them due to the side effects. They are familiar with the excessive urination and thirst that often occur with steroids. What’s really critical to treatment success for these acute flares is a treatment option that is well-tolerated and has good acceptance and compliance by the owner. When an owner sees how quickly Apoquel helps reduce the itchiness and inflammation of their pet’s allergy flares without the common side effects of steroids that include polyuria, polydipsia, and polyphagia, several critical keys to success occur: peace of mind (and, thus, the bond with the pet restored), increased trust in the veterinarian, and a positive experience. So although these guidelines are a good starting point, they reflect the experiences in controlled clinical trials, and what they can miss is that individual pet and owner experience. This is where Apoquel is an ideal targeted therapy for the quick management of flares of allergic and atopic dermatitis. The side effects of Apoquel reported most often with short-term use are vomiting and diarrhea.
Our clinic has gotten so many new clients just because we can effectively treat allergic dermatitis. It has helped grow our business.—Dr. Hebert
Patricia Stark: In your opinion, what are the medical benefits of using Apoquel as a first-line therapeutic?
Dr. Eeg: I’ve had the great opportunity to use Apoquel for over 5 years in private general practice, and I can tell you that it is the first-line medication we use for allergic itch and inflammation. People want their pet better soon; they don’t want to wait. Having Apoquel allows me to get the pet more comfortable starting within 4 hours.
Dr. Hebert: It is important to also look at the client impression. It is 2019, and the online community rules at this point. There are Facebook pages that are meant to be community support pages where people can say, “My dog has this horrible, itchy skin disease, and nothing is working. Can anybody recommend somebody in the area that can help?” For many of my clients and patients, the experience with Apoquel is different from the experience with antihistamines and steroids. And that’s what people post back on Facebook. Our clinic has gotten so many new clients just because we can effectively treat allergic dermatitis. It has helped grow our business.
With the effective relief of allergic pruritus provided by Apoquel, the patient can start getting a full night’s sleep. And when I haven’t warned clients about that, I’ve had some people say, “Well, I think there’s some kind of amphetamine effect in this or something.” But it’s just because for the first time in weeks, the dog gets a good night’s sleep. It has been so long since they saw their dog normal and without pruritus.
Patricia Stark: Despite the guidelines, antihistamines and steroids are still used as first-line therapy by many veterinarians, especially early in disease. Why is this, and what are the real consequences of not using Apoquel as a first-line therapeutic?
Dr. Hebert: There is this presumption that veterinarians feel the client wants the least expensive option first, and it’s a disservice to the patient and the owner. They are looking to us as veterinarians for a recommendation on what is right for their pet. It’s our responsibility to consider efficacy and safety when making a recommendation for best medicine for a patient.
Dr. DeBoer: Sometimes veterinarians think they should start with “the most conservative treatment,” which is antihistamines. But that is starting with something that doesn’t work, because we know antihistamines don’t have much effect against pruritus in dogs.
Dr. Eeg: Having a first-line drug like Apoquel to improve that pet’s quality of life and reduce the clinical signs of both the pruritus and inflammation is critical. One of the main benefits I see is staff having a much better experience. When a client calls saying their dog, who’s been on steroids, is peeing in the bed at 2 am, they are not very happy. The most common side effects of Apoquel in short-term clinic trials were vomiting and diarrhea, which occurred no more commonly than in placebo-treated dogs. So for my clients, the experience with Apoquel tends to be better than with steroids or antihistamines.
Patricia Stark: Now that Cytopoint has a label indication for use in dogs with allergic dermatitis, is it okay to use it as a first-line therapy in place of Apoquel?
Dr. McKay: To clarify, first-line use of Apoquel means that we are using Apoquel both during the diagnostic workup as well as for an acute flare, whether that is a food allergy flare, a flea infestation, or a high pollen count leading to a flare. And Apoquel really is the best first-line option for most dogs during the allergy workup. Once you stop Apoquel, within a few days, it is out of their system, so we can quickly see if some of our treatment trials have been helpful. With Cytopoint, that is different, as it has effects for 4 to 8 weeks, so you cannot stop and start itch control as you can with Apoquel. However, there are specific scenarios in which Cytopoint is the best first-line option—for example, for dogs that weigh <6 lb and are too small to dose accurately with Apoquel, are younger than 1 year, or are difficult to pill or for owners who may have physical limitations and may struggle to administer oral medications.
No cause-and-effect relationship has been established between administration of Apoquel and development of neoplastic conditions. —Dr. Cleaver
Patricia Stark: Some clinicians question the association between administration of a Janus kinase (JAK) inhibitor, like Apoquel, and cancer. What can we learn from the package insert?
Dr. Cleaver: The Apoquel label states in the warning section that the drug may exacerbate neoplastic conditions. In the precautions section, it says that dogs on Apoquel should be monitored for development of neoplasia. These statements appear as a result of the classification of Apoquel and not as a result of any specific studies that have shown exacerbation of neoplasia. No cause-and-effect relationship has been established between administration of Apoquel and development of neoplastic conditions.
In the pivotal clinical study of atopic dermatitis, which went for almost 4 months, and Continuation Field Study, which monitored some dogs for as many as 600 days, low numbers of different types of cancers were reported. So it’s there on the label for veterinarians to be aware of. Although these conditions were observed while the dogs were on Apoquel, it is always important to remember that observation does not always equate to causality.
Patricia Stark: What are the latest science and research findings on JAK inhibitors and cancer? Can we learn anything from the experience with JAK inhibitors in humans and their association with the risk for development of neoplasia?
Dr. London: There have been a lot of misconceptions regarding JAK inhibitors and their role in cancer. JAK inhibitors have been shown to be very valuable medications on the human side. The most commonly used JAK inhibitor, tofacitinib, has been studied for over 5 years, with thousands of patients followed. It is clear that there is absolutely no increased risk for cancer in patients that received tofacitinib for several years. Broader immunosuppressive agents that truly and actively suppress the immune system in humans have been linked to certain types of cancers; those cancers are usually caused by viruses, not by nonviral causes. There is no definitive evidence that any of the JAK inhibitors used to treat humans with various autoimmune or immune-dysregulatory diseases contribute to an increased cancer risk.
In fact, we’ve conducted some trials combining Apoquel with chemotherapy to look for additive benefits, and there are ongoing studies looking at a potential for Apoquel to treat cancer, because we know that certain JAK-signaling pathways are dysregulated in cancer and contribute to continued tumor growth. In these circumstances, inhibition of the JAK signaling pathways may be helpful in treating some cancers.
Many canine patients that are at increased risk for cancer are also at increased risk for atopic dermatitis. For example, golden retrievers have a significantly increased risk for cancer, and they are one of the breeds that commonly gets atopic dermatitis. So when comparing cancer rates, you have to consider the cancer rate in golden retrievers that are on Apoquel versus those not on Apoquel; I suspect you would see no difference.
Dr. Gonzales: We are seeing an explosion of studies evaluating the presence of genetic alterations in JAK enzymes in human malignancies. Some studies have found activating mutations in JAK enzymes that cause the enzyme to be turned on continuously. These defects may be causing the formation of certain types of tumors in humans. Because of these findings, human and animal health researchers have been evaluating whether JAK inhibitors could be useful as treatments in certain types of cancers in which JAK pathways may be dysregulated.
The cancer process is very complex. Tumor formation can happen over many years, and different mutations and changes have to occur for neoplasia to develop. Chronic inflammation can contribute to the neoplastic process; it creates a microenvironment that can promote some of the changes that might be pro-tumor forming; so, some of these animals that have a predisposition to chronic inflammation, like dogs with chronic allergic dermatitis, may be inherently predisposed to neoplasia as well.
Dr. Eeg: In the 5 years I’ve used Apoquel, there has been no increase in any specific type of tumor in our patients that are on Apoquel as compared with dogs that don’t have allergic dermatitis.
We now have 5 years of experience with Apoquel, and a review of pharmacovigilance data showed no unexpected findings.—Dr. Bateman
Patricia Stark: Why do we see reports of neoplasia in dogs on Apoquel?
Dr. London: We should first recognize that the cancer rate in the general dog population, regardless of whether they’re on Apoquel, is extraordinarily high—higher than it is in people. There are almost 400 cases of cancer per 100,000 dogs per year, and that compares to 300 cases of cancer per 100,000 people. So, at some point, if you are treating a patient for a long time, it has a very high likelihood of getting a tumor. In fact, if a dog lives to 10 years or older, there is a 50/50 chance that it will get a cancer. So if you are following these dogs long-term, chances are you are going to see cancers develop regardless, and it is probably unrelated to medications that have been administered.
Dr. Hebert: Veterinarians need to be aware that there is a lot of misinformation out there about Apoquel, as well as Cytopoint. There are Facebook pages, unfortunately, devoted to the erroneous belief that it causes cancer. It’s usually just a lack of education. For example, a pet owner whose 10-year-old dog developed cancer months after starting Apoquel may consider Apoquel as the cause of the dog’s cancer, when in fact it’s merely coincidence.
Dr. Bateman: I think people are cautious because this is a totally new class of drug in veterinary medicine. We now have 5 years of experience with Apoquel, and a review of pharmacovigilance data showed no unexpected findings. It’s important that we report any possible adverse events to develop that pool of knowledge, because our responsibility as specialists and veterinarians is to evaluate the pharmacovigilance data. Without that data, we are unable to provide that evidence-based perspective.
Dr. DeBoer: We had the same conversation when cyclosporine became available for atopic dermatitis. We use azathioprine, a very immunosuppressive drug, all the time, and we don’t think about it causing cancer. But we do understand it’s difficult for owners when their pets get cancer, and they may be looking for something to blame.
Dr. Eeg: When clients bring in “Dr. Google” information, they are looking for the veterinarian’s response to that information and level of confidence in the drug. Apoquel is one of those drugs that I have great confidence in, especially after hearing all of the experts align here. The simple answer is that you let them know you have reviewed the data on Apoquel and have confidence in the efficacy and safety profile of this compound in treating dogs with allergic and atopic dermatitis.
The data continue to point toward an immunomodulatory effect rather than an immunosuppressive effect. —Dr. DeBoer
Patricia Stark: Apoquel is classified by the FDA as an immunosuppressive drug. Why is that the case when similar human drugs are categorized as JAK inhibitors?
Dr. Cleaver: When Apoquel was approved in 2013, the standard list of pharmacologic classes didn’t include JAK inhibitors, so the Center for Veterinary Medicine (CVM) at the FDA required Zoetis to categorize Apoquel as an immunosuppressant. Following that, several JAK inhibitors were approved in human medicine, and over time, they became established as a class.
Dr. Gonzales: We were very careful when we chose the dose and regimen for Apoquel. Our goal was to partially inhibit cytokines that were involved in pruritus and inflammation and associated with allergic skin disease while maintaining normal function of key immune responses such as T-cell function. To accomplish that, we incorporated into our testing funnel a variety of in vitro screens, and we also conducted some key in vivo studies, such as our vaccine-response study. The goal of those experiments and studies was to assess immune function during Apoquel treatment in the dog. We felt the results from those experiments and in vivo studies, which often used exaggerated doses of Apoquel, suggested that animals could still mount an appropriate immune response, indicating maintenance of T- and B-cell functions and ensuring that the label dose would not affect these immune reponses.
Dr. DeBoer: I found it interesting that in vitro, when dog lymphocytes are cultured with different concentrations of Apoquel and the therapeutic concentrations are used, there is no inhibition of that cell function at all.1 You have to use 10 or more times the therapeutic concentrations to have any effects on dog lymphocytes in vitro. The data continue to point toward an immunomodulatory effect rather than an immunosuppressive effect.
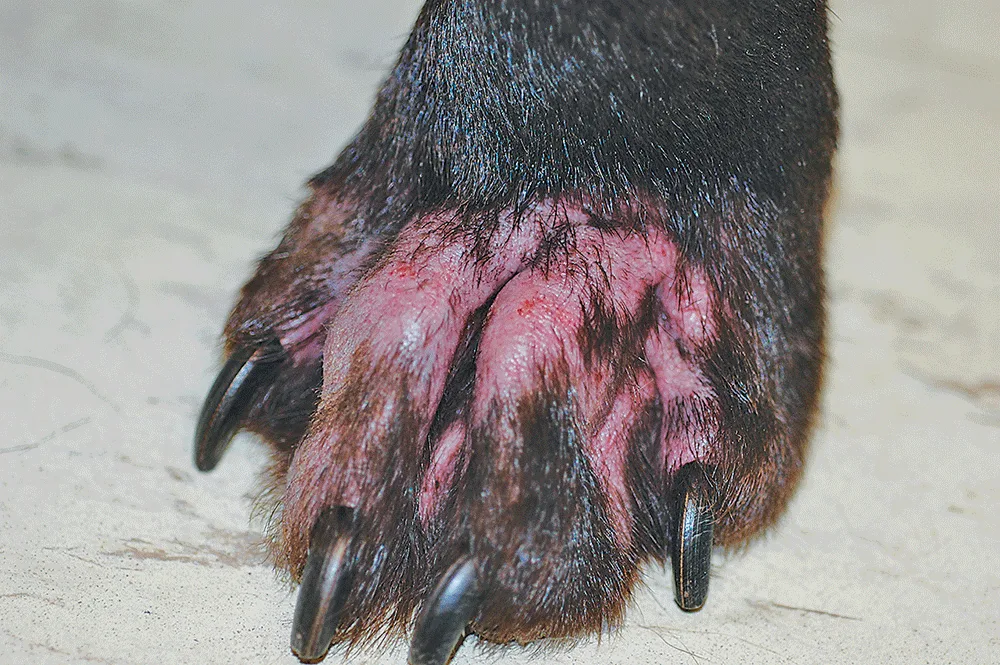
Patricia Stark: In veterinary dermatology, which drugs are typically used as immunosuppressive agents, and what sort of disease conditions are they used to treat?
Dr. Bateman: Immunosuppressive agents are primarily used for the treatment of autoimmune and immune-mediated dermatologic disease (eg, pemphigus vulgaris, pemphigus foliaceus). Treatment of autoimmune disease is not on the Apoquel label; instead, we rely on medications such as corticosteroids, cyclosporine, azathioprine, and a number of other broader immunosuppressive agents to suppress the abnormal immune response in these conditions. Immunosuppression is really not our goal when treating allergic skin disease and atopic dermatitis; instead, our goal is immune modulation and downregulation of the mediators of itch and skin inflammation.
A treatment like Apoquel is not contributing to secondary infection but likely helping to prevent it. —Dr. McKay
Patricia Stark: The risk for secondary infections of different organ systems, including the urinary tract, respiratory tract, and skin, is increased in patients on immunosuppressive therapies. What has been the reported incidence of infections in dogs on Apoquel?
Dr. McKay: There is a perception that immunosuppressive treatments for atopic dermatitis could increase the incidence of skin infections, but I believe in general that controlling the inflammation of allergic dermatitis with a drug like Apoquel actually helps prevent flares of bacterial and yeast skin infections. Studies indicate that in dogs with atopic dermatitis, as many as two-thirds will develop pyoderma and one-third will develop Malassezia spp dermatitis.2 In some of the studies that have examined the incidence of pyoderma and Malassezia spp dermatitis in dogs on Apoquel, the authors all noted low rates of pyoderma of about 9% to 11%. Looking at yeast dermatitis, only about 2% to 4% of dogs in these studies developed a Malassezia spp infection.3-5 This suggests that a treatment like Apoquel, although it might be categorized by the FDA as an immunosuppressive therapy, is not contributing to secondary infection but likely helping to prevent it.
There was a study performed in 55 dogs receiving at least 6 months of Apoquel therapy to see if they were developing subclinical bacteriuria or actual evidence of clinical UTIs; none of the dogs developed UTIs over the duration of the study.6 This is really encouraging that the use of Apoquel was not associated with increased risk for secondary urinary infections.
Patricia Stark: What information is available that helps veterinarians understand the long-term safety of Apoquel?
Dr. Cleaver: The package insert has quite a bit of information on long-term safety. We conducted a 6-month marginof-safety study in which we tested dogs with 1×, 3×, and 5× the target dose. In this study, Apoquel was found to be safe in the target population of animals over 12 months of age. In the Continuation Field Study, we worked with CVM to allow use of the drug for long-term maintenance therapy. A total of 239 dogs were part of the study, and the average duration of Apoquel administration was over a year. These dogs were receiving their regular veterinary care, and other health conditions were occurring; all this information was collected and reported and contributes to our understanding of the long-term safety of Apoquel.
Dr. McKay: Apoquel was one of the first drugs the CVM allowed for compassionate use through the Continuation Field Study because of the fact that it was such a game changer for these dogs. During that study, those dogs were living their normal lives. We monitored them very closely; in fact, we saw them every 3 months to complete a physical exam, CBC, serum chemistry profile, and urinalysis. We saw excellent safety, as well as efficacy, during this period of time. When our patients went on Apoquel, most of them had never been able to find anything that controlled their disease prior to this, and their quality of life was dramatically improved when we had the opportunity for this compassionate use of Apoquel in the Continuation Field Study.
Patricia Stark: How should veterinarians monitor dogs on Apoquel maintenance therapy?
Dr. Bateman: We have a progress check and do routine blood work on our Apoquel patients every 6 to 12 months.
Dr. DeBoer: Do we need to be doing, or recommending, urine cultures in patients on Apoquel? One study suggests this isn’t necessary.6
Dr. Bateman: I don’t routinely do urine cultures on dogs that are on Apoquel, but I will if the animal is on steroids.
Dr. McKay: Based on my personal experience, I recommend exams and monitoring, including CBC, serum chemistry profile, and urinalysis, every 6 to 12 months for my patients that are chronically on Apoquel.
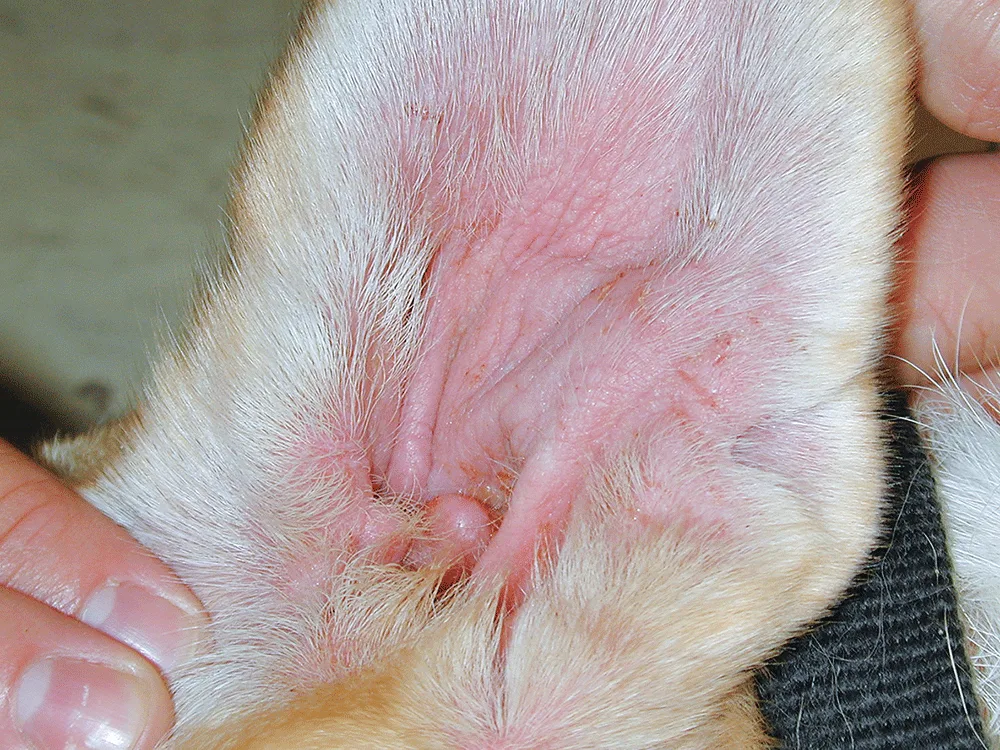
Patricia Stark: What have we learned from postapproval pharmacovigilance?
Dr. Cleaver: More than 900 million pills have been administered and more than 6 million dogs treated over the past 5 years. After more than 5 years of postapproval pharmacovigilance data, we have found that what was reported during the clinical trials on the label is consistent with what we are seeing now.
Dr. McKay: I have probably treated well over 1,000 dogs with Apoquel over the last 10 years (preapproval clinical trials and postapproval); the safety is the same as I saw initially. My overall impression after a decade is that this is generally very well-tolerated.
Dr. Eeg: We saved dogs that were otherwise going to be euthanized, because they were having such significant negative quality-of-life components that the owners were not going to continue, and many of the dogs had reached a point where their continued treatment options were nil. So I think, when you look at the long-term effects, really you have to look at the quality-of-life effects.
Patricia Stark: Do you believe Apoquel plays a role in long-term management of dogs with atopic dermatitis?
Dr. DeBoer: It certainly does. Longterm, we have to find something that is going to manage the itch and inflammation. In addition, we look to manage any known flare factors and bathe these dogs frequently.
Dr. McKay: I tell my clients they are going to need an anchor treatment—something that can control all or most of the pet’s atopic dermatitis. For most patients, Apoquel is able to control itch and inflammation and serve as a great anchor treatment. That’s how I see Apoquel now in long-term management; it is a game changer for so many dogs with allergic dermatitis, specifically atopic dermatitis, because it can act as a sustainable anchor drug long-term.
Dr. Eeg: One of the long-term treatment opportunities with Apoquel is allowing clients to have some medication on hand so that if there is a flea event, they can start the pet on Apoquel and control the itch and inflammation until flea treatment is restarted. We’d like all of our patients to be on an isoxazoline, but that’s sometimes not possible, and owners may be hesitant to continue treatment as needed because they don’t see fleas or don’t perceive fleas to be a constant year-round problem. Also, consider food allergic dogs; when they have a dietary lapse or even when the owner is unable to strictly feed a hypoallergenic diet, Apoquel can help provide control of clinical signs of food allergy.
Patricia Stark: What should veterinarians do if a dog that has been well-controlled on Apoquel is having increased signs of allergy and the client doesn’t believe the current treatments are working?
Dr. Bateman: Apoquel is an excellent choice for short- and long-term control of allergic and atopic dermatitis, but that doesn’t mean flares can’t happen. If an owner reports increased signs of itch, there may be other factors at play. In our clinic, we get that patient in and check for infection (eg, parasitic, bacterial, fungal). In my experience, the antipruritic activity of Apoquel may not be quite as good when infection is present, so that may be causing the breakthrough until the infection is addressed. Dogs can develop food allergies over time, so I will question the owner about diet changes, new treats or flavored medication, and bowel habits; if anything has changed, we perform an elimination diet trial.
There are certain times of the year when the environmental allergen load is higher—for example, when tree pollen counts are high during late winter to early spring. Owners will often know this and be prepared; we need to add another temporary treatment option for those animals during those periods, such as bathing or Cytopoint.
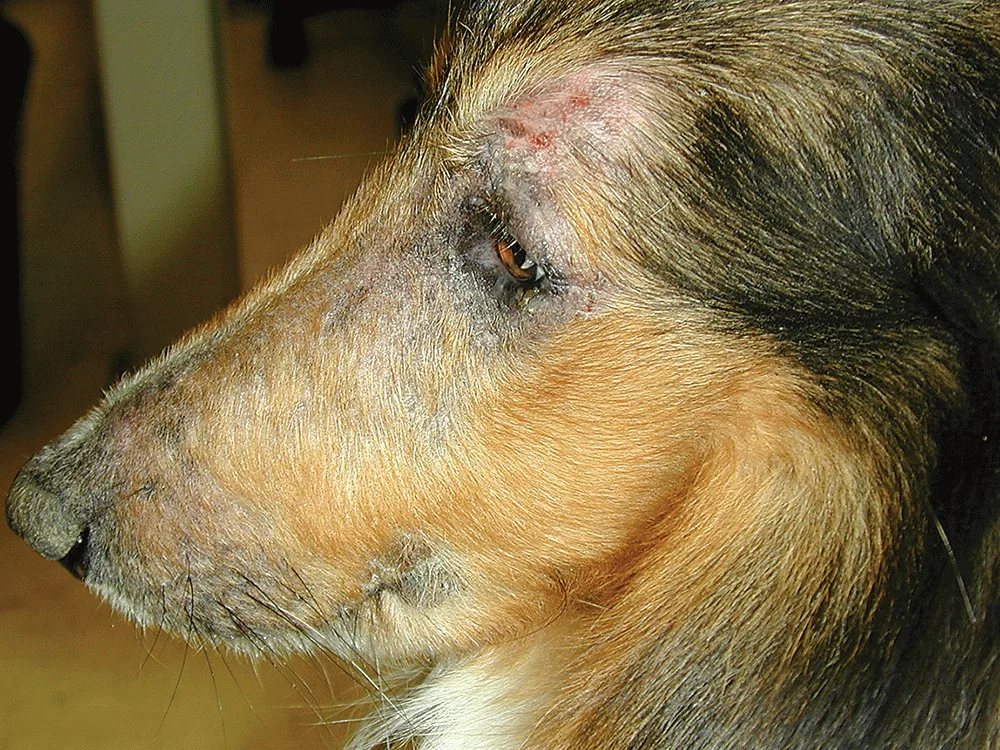
Patricia Stark: What other points are important to consider for a dog having a flare of allergic pruritus?
Dr. Hebert: With the safety of using Apoquel long-term and the rapid onset of action, we can figure out a dog’s seasonality. Within 24 hours of stopping Apoquel, we will know if a dog needs more, and the owner can give it to start providing relief within hours. Because we can stop and start Apoquel so precisely and use it for as short or as long as the patient needs, we can help them through their most severe allergy season and help prevent those itch flares and skin or ear infections.
Dr. DeBoer: I can’t emphasize enough how important it is to keep infections under control, because very often that ends up being the reason Apoquel or another medication isn’t working so well.
Patricia Stark: Is there any evidence to suggest Apoquel also has antiinflammatory effects in allergic dermatitis?
Dr. Gonzales: We have studied Apoquel’s mechanism of action, and we know it inhibits a variety of cytokines that are involved in inflammation, most notably IL-2 and IL-6. It also inhibits IL-4 and IL-13, which tend to be associated with allergic responses and activate cell types such as mast cells, eosinophils, and B cells; those cells can release a lot of proinflammatory mediators associated with allergic dermatitis. We also know Apoquel inhibits IL-31. Although this cytokine is attributed mostly to pruritus, we are learning that it has the potential to activate many chemokines and proinflammatory mediators. So, inhibiting that cytokine probably contributes to some of its anti-inflammatory effects.
In addition, we have studied the effects of Apoquel in a variety of laboratory models of allergic skin disease, in addition to clinical trials. One of the endpoints we tend to look at in laboratory models is erythema, which is a sign of skin inflammation. In laboratory dogs treated with Apoquel, we’ve seen great improvement in skin condition and reduction of erythema. In comparative laboratory studies, we have found that Apoquel works just as effectively as a steroid.
It’s clear that there’s not only an antipruritic effect, but also an effect on inflammatory lesions. —Dr. DeBoer
Patricia Stark: Have there been any studies that demonstrate Apoquel’s effectiveness in reducing lesions associated with allergic and atopic dermatitis?
Dr. DeBoer: We have good, standardized, validated ways of assessing lesions and pruritus in allergic and atopic dogs. We know from the clinical field studies involving dogs with spontaneous atopic dermatitis that Apoquel not only rapidly and significantly reduced the itch, but the lesion scores as well. In follow-up studies when Apoquel was compared head-to-head with either cyclosporine or corticosteroids, we saw pretty much equivalent reduction in the inflammatory lesions with all of those drugs. It’s clear that there’s not only an antipruritic effect, but also an effect on inflammatory lesions.3-5
Patricia Stark: Are there any circumstances in which steroids may be preferred over Apoquel?
Dr. McKay: In general, other than for chronic ear cases, I would only reach for steroids in rare cases when efficacy was not good with other treatments or in the case of adverse effects from other treatments. If a dog is younger than a year and Apoquel is not appropriate due to its age limitation, I would turn to Cytopoint, which now has a label claim for allergic and atopic dermatitis. You could consider steroids there, but they are really not ideal in growing dogs.
Dr. DeBoer: With ear disease, one of the major issues is swelling in the ear canal, and sometimes that swelling really needs corticosteroids. However, topicals are also available to treat that. Also, in cases of severe pododermatitis when there is a lot of swelling in the feet, steroids may be needed initially.
Conclusion
Allergic dermatitis has been a frustrating area of practice for clinicians, but it has been changed by Apoquel. Apoquel has been shown to provide fast, effective, and safe management of flares of allergic and atopic dermatitis. Patients enjoy a greater quality of life because of the immediate relief of pruritus and inflammation. The efficacy of Apoquel has helped increase clients’ trust in their veterinarian as a caretaker for their pet, and there’s still more to learn about what Apoquel can offer beyond treating allergic itch and inflammation—like the potential benefits in the treatment of cancer. Apoquel has become an indispensable asset in the treatment and management of allergic and atopic conditions in veterinary dermatology.
IMPORTANT SAFETY INFORMATIONDo not use APOQUEL® (oclacitinib tablet) in dogs less than 12 months of age or those with serious infections. APOQUEL may increase the chances of developing serious infections, and may cause existing parasitic skin infestations or pre-existing cancers to get worse. APOQUEL has not been tested in dogs receiving some medications including some commonly used to treat skin conditions such as corticosteroids and cyclosporine. Do not use in breeding, pregnant, or lactating dogs. Most common side effects are vomiting and diarrhea. APOQUEL has been used safely with many common medications including parasiticides, antibiotics and vaccines. For more information, please see the full Prescribing Information.
CVM = Center for Veterinary Medicine, JAK = Janus kinase, UTI = urinary tract infection