Canine Hemangiosarcoma
Timothy M. Fan, DVM, PhD, DACVIM (Oncology, Internal Medicine), University of Illinois at Urbana–Champaign
Canine hemangiosarcoma (HSA) is a highly malignant solid tumor that arises from the malignant transformation of endothelial cells or from neoplastic bone marrow progenitor cells of hemangioblast differentiation.1-3
Background & Pathophysiology
HSA can develop in any vascular organ or tissue but is most commonly found in the spleen (≈50%), right atrium and/or auricle (≈25%), and skin or SC tissue (≈15%; Figure 1).4 Most dogs diagnosed with HSA are geriatric, with a predisposition observed in German shepherd dogs, golden retrievers, and Labrador retrievers.5-7 Splenic HSA is a common splenic malignancy and is accompanied by life-threatening complications (ie, hemoabdomen and distant metastases).8-11 The mode of metastatic spread varies for patients with HSA that involves the abdominal visceral organs (eg, spleen, liver, kidneys). Regional dissemination of disease in the abdominal cavity or retroperitoneal space is enabled by the local deposition of tumor cells following primary tumor rupture, but distant metastasis requires hematogenous circulation, vascular entrapment, and successful colonization of detached tumor cells. Common metastatic sites include the liver, omentum, mesentery, and lungs.7,12 HSA also tends to metastasize to the CNS.13,14
Although HSA involving visceral organs is typically uniformly malignant, the biologic behavior of cutaneous HSA varies in aggressiveness and depends on the extent of localized (dermal, hypodermal/SC, or muscular) invasion. In dog breeds with short hair and minimal pigmentation, sun exposure (ie, actinic induction) is a risk factor for superficial cutaneous HSA.15,16 This dermally confined variant of HSA tends to be less aggressive due to lack of subdermal penetration and reduced capacity for establishing distant metastases as compared with HSA that involves deeper adnexal structures (including tissues of the subcutis and muscle).15,17-21


Primary noncutaneous HSA involving the head of the spleen (A) and the right auricle (B, arrowheads). Images courtesy of Laura Garrett, DVM, DACVIM (Oncology), and Louis-Philippe de Lorimier, DVM, DACVIM (Oncology)
History
Many dogs with HSA remain clinically normal for relatively long periods of time (ie, months) during malignant progression. An HSA diagnosis involving visceral organs is often precipitated by sudden life-threatening signs related to hemodynamic collapse following acute, nontraumatic organ rupture and consequent hemoabdomen. In affected dogs that do not experience life-threatening hemorrhage, clinical signs of nonspecific lethargy may fluctuate and an episodic pattern related to intermittent hypovolemia associated with acute third-space blood loss may be exhibited.
Clinical Signs
Clinical signs are nonspecific and depend on the anatomic location of the primary tumor as well as the magnitude and severity of resultant hemorrhage and hypovolemic shock. Common clinical signs include acute-onset lethargy, weakness, collapse, pale mucous membranes, delayed capillary refill time, tachycardia, cardiac arrhythmias, and poor pulse quality. If blood loss is self-limiting, these hemodynamic perturbations are not imminently life-threatening; clinical signs may be episodic in nature and a full clinical recovery may follow within days. However, when hemorrhage is severe, hemodynamic collapse and sudden death are possible.
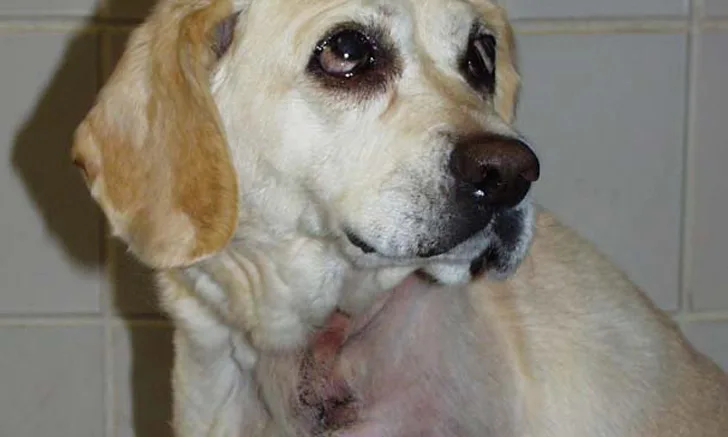
A large space-occupying mass may be palpable with visceral organ (splenic or hepatic) HSA. In addition, tumor rupture can result in abdominal distention and ballottement of a fluid wave secondary to hemorrhagic effusion. With cardiac HSA, malignant arrhythmias (eg, ventricular premature contractions), muffled heart sounds, venous congestion (eg, jugular pulses, facial edema, hepatic venous congestion with effusion) associated with right-sided heart failure, and signs compatible with cardiac tamponade may be observed. Primary HSA lesions involving the superficial dermis may appear as well-defined blood blisters. HSA from the SC and IM tissues may appear as large and firm or fluctuant masses (Figure 2); overlying skin may have extensive ecchymosis, swelling, discoloration, and ulceration.

Primary HSA involving SC tissue. Image courtesy of Louis-Philippe de Lorimier, DVM, DACVIM (Oncology)
Diagnosis
Although HSA can be presumptively diagnosed based on multiple clinical and physical findings and patient signalment, baseline diagnostic tests (see Baseline Diagnostic Tests) should be considered in patients that have probable HSA. Detailed images of HSA lesions arising from visceral organs, SC tissue, and deeper muscle structures can be acquired with advanced imaging modalities (eg, CT; Figure 3).



CT images of primary splenic HSA (A, arrowhead), SC HSA (B, arrowhead), and deep-muscle HSA (C, arrowhead). Figures courtesy of Louis-Philippe de Lorimier, DVM, DACVIM (Oncology)
Definitive diagnosis of HSA requires microscopic identification of tumor cells through cytology or histopathology. Due to the poorly exfoliative nature of mesenchymal tumors coupled with the hemorrhagic nature of HSA, fine-needle aspiration cytology often produces samples of low cellularity with rare identification of malignant cells (Figure 4). Cytologic examination of hemorrhage effusions is not typically helpful or diagnostic for HSA because of the low numbers of neoplastic cells admixed with a large volume of blood. Biopsy is suggested for definitive diagnosis because benign lesions (ie, splenic hematoma) can have clinical presentations (ie, splenic mass with associated hemoabdomen) similar to malignant HSA. Excisional biopsy is preferred because it is diagnostic and therapeutic. All resected tissue samples should be evaluated for histologic features of malignancy and immunohistochemically stained for endothelial markers (eg, CD31).
BASELINE DIAGNOSTIC TESTS
Test results are listed in parentheses.
CBC (anemia, schistocytes, thrombocytopenia)
Serum chemistry profile (hypoproteinemia secondary to blood loss, liver enzyme elevation)
Coagulation panel (elevated prothrombin time/partial thromboplastin time secondary to disseminated intravascular coagulation)
Abdominal and thoracic radiography (mass effect or pulmonary metastases; Figure 5)
Echocardiography (right auricle mass effects; Figure 6)
Abdominal ultrasonography (mixed echogenic mass lesions involving visceral organs)
Additional radiography studies of affected anatomic sites (Figure 7)
Advanced imaging modalities (eg, CT)

FIGURE 4A
Cytology showing malignant mesenchymal cells with multiple criteria for cellular malignancy (eg, anisocytosis, anisokaryosis; A); histopathology of the splenic mass demonstrating malignant spindle cells forming haphazard and disorganized vascular channels (B) and strong positivity for the expression of CD31 (PECAM-1), which is represented by brown membranous staining of HSA cells and confirms that malignant cells are of endothelial origin (C). Images courtesy of Anne Barger, DVM, MS, DACVP, and Jonathan Samuelson, DVM, DACVP
Treatment & Management
Effective management of HSA is difficult, as therapeutic options (eg, surgery for cardiac HSA) can be associated with high morbidity, and palliative treatments (eg, radiation therapy) result in only marginal improvements in overall survival times. However, based on the biologic behavior of most HSA cases, comprehensive treatment plans should include a combination of localized and systemic therapeutic strategies except in cases of superficial dermal HSA, which can be treated with surgical resection alone. In patients with cardiac HSA, local treatment performed by a skilled surgeon can be attempted with the aim of resecting or cytoreducing primary tumors that involve the atrium or auricle; however, morbidity (and mortality) can be associated with these interventions. Pericardiectomy may be performed to mitigate the development of cardiac tamponade and associated hemodynamic compromise.22,23 Ionizing radiation therapy can also be used for localized treatment of SC- or muscle-invasive HSA, in which effective surgical intervention is anatomically infeasible.24 Although localized interventions (ie, surgery, radiation) are frequently palliative in nature and have the potential to control clinical signs associated with hemorrhage and pain, definitive efficacy of these treatment options remains speculative and prospective clinical trials are needed. Splenectomy followed by adjuvant systemic chemotherapy remains the standard of care for splenic HSA. In most anatomic forms of HSA, except superficial dermal involvement, regional and distant metastases can develop rapidly, and most dogs succumb to disseminated disease progression. The highly metastatic nature of HSA has been thoroughly documented in dogs with splenic HSA, in which a median survival time of 1.6 months is expected in dogs treated with splenectomy alone.11
Chemotherapeutic approaches can support a marginally to modestly improved survival time (4-8 months) of dogs with HSA (mostly splenic) treated with systemic, maximum-tolerated-dose chemotherapy, primarily with a doxorubicin backbone protocol. Attempts to identify adjuvant and/or alternative systemic therapies (eg, metronomic chemotherapy, receptor tyrosine kinase inhibitors) to extend survival times have been largely unsuccessful.12,25-27 Novel strategies that include administration of bispecific drug conjugates, inhibition of β-adrenergic signaling, and chemoimmunotherapy with dendritic cell vaccination and low-dose doxorubicin therapy are promising and under investigation28-30; however, these strategies are not in mainstream use for dogs with HSA.
Dogs with HSA may also clinically benefit from alternative complementary therapies such as Yunnan Baiyao and a commercially available proprietary polysaccharopeptide (PSP) extract. Yunnan Baiyao has been recently investigated for its potential procoagulant properties.31,32 In healthy beagles, Yunnan Baiyao has been shown in some studies, but not in others, to increase the strength of blood clot formation as measured by thromboelastography.31,32 In dogs presented with presumed HSA, Yunnan Baiyao may improve postoperative surgical outcomes but requires future prospective studies to define its role in HSA management. Similar to Yunnan Baiyao, the PSP extract is a mushroom extract believed to act as an immunostimulant and has shown some potential in delaying the onset of abdominal metastases following splenectomy in a small pilot study.33 Although initial results for the PSP extract are promising, additional and larger prospective studies evaluating its clinical benefit have not been published. The PSP extract’s role in HSA management therefore remains incompletely defined.
Clinical Follow-Up & Monitoring
Early disease screening and tumor burden monitoring through routine radiography and sonography or molecular diagnostics can provide information on HSA disease status for pet owners and can improve clinical management of initial or recurrent HSA lesions. This is supported by superior survival outcomes in dogs diagnosed with early- versus late-stage disease.11,34,35 Because HSA rapidly progresses, follow-up examination including blood work and monitoring (via thoracic radiography and abdominal ultrasonography) should be routinely performed, with follow-up scheduled depending on stage of disease, speed of disease progression, clinical signs, and owner compliance. Dogs treated with splenectomy and systemic chemotherapy that remain clinically stable should be re-examined every 8 weeks; this allows for local and/or systemic intervention when recurrent and/or metastatic disease is incipient.