Biopsy of Suspected Injection-Induced Sarcoma in Cats
Profile
DEFINITIONSoft tissue sarcomas that occur in the subcutis of the dorsal neck/interscapular area, flank/paralumbar area, dorsolateral thorax, and femoral musculature have been termed injection site sarcomas or vaccine-associated sarcomas due to their anatomical location at common sites of subcutaneous injection (Figure 1).
SIGNALMENTAge and Range. Injection site sarcomas occur in younger cats than do other types of sarcomas. Peak occurrence is 6 to 7 years of age. Injection site sarcomas may appear months to years after vaccination or other injections.
Genetic Implications. Injection site sarcomas have been found in related cats, suggesting a genetic predisposition.
CAUSES/RISK FACTORSMost injection site sarcomas have been associated with vaccines-particularly rabies and FeLV-but some have also been associated with vaccination for feline panleukopenia, herpesvirus, and calicivirus. Risk increases with the number of vaccines given at a particular site. Injection site sarcomas have also arisen at the site of antibiotic administration, subcutaneous fluid administration, long-acting corticosteroid injection, or lufenuron injection.
PATHOPHYSIOLOGYFibrosarcomas are the most commonly diagnosed histologic type of injection site sarcoma, but steosarcoma, malignant fibrous histiocytoma (histiocytic sarcoma), and other sarcoma types have also been described.
Injection site sarcomas are often associated with an inflammatory infiltrate, primarily macrophages that are frequently reported to contain bluish "foreign matter," and may include giant cells. In one study, aluminum was associated with this inflammation, leading to speculation that aluminum-containing adjuvants in vaccines may cause inflammatory changes that lead to sarcoma formation. Another study showed an increasing risk for sarcoma formation with the use of killed vaccines. Because adjuvants are used with killed vaccines and seem to be responsible for much of the inflammation, some investigators have suggested the preferential use of modified-live vaccination. However, sarcomas have also been reported to occur with administration of these vaccines.
The current thinking is that certain cats respond to inflammatory changes in a manner that predisposes them to sarcoma formation. This is supported by the discovery of cats that have developed sarcomas at more than one injection site and the finding of injection site sarcomas in related cats.
CLINICAL SIGNSThe occurrence of a mass at a site commonly used for subcutaneous or intramuscular injections should alert the clinician to the possibility of an injection site sarcoma.
Diagnosis
Biopsy samples should be collected from a mass or from a vaccination reaction that persists or starts to increase in size. In one study, most reactions to rabies or to FeLV vaccination resolved within 2 to 3 months; some oncologists believe that a biopsy sample should be obtained when a vaccine reaction persists longer than 1 month. To maximize success and minimize side effects of treatment, a pretreatment biopsy is recommended to guide therapy.
As soft tissue sarcomas grow, they compress a cuff of tumor cells to form a pseudocapsule, thereby giving the false impression that the sarcoma is encapsu lated. Furthermore, injection site sarcomas often contain necrotic areas. Thus, a biopsy, particularly a needle biopsy, should include not only the periphery of the tumor but also deeper tissues, and several samples should be taken. When planning the path of the biopsy needle, consider the effect on future surgery or radiation therapy, as the biopsy tract may seed tumor tissue.
STAGINGStaging procedures should be completed to establish the health of the patient and to delineate the extent to which the sarcoma may have spread before the pretreatment biopsy was obtained. Staging should include a minimum database (complete blood count, serum chemistry profile, urinalysis, FeLV and FIV serologic testing, serum T4, thoracic radiographs), thorough history of previous vaccinations and injections, and abdominal ultrasonography. Enlarged regional lymph nodes should be aspirated for cytologic evaluation.
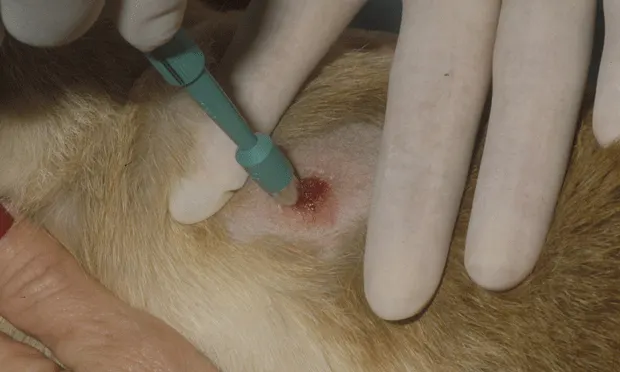
MORE-DESIRABLE BIOPSY TECHNIQUESNeedle-Core Biopsy. A needle-core biopsy is safe and rapid and can be performed with light sedation and a local anesthetic block. Care should be taken to avoid damage to the small tissue fragments during handling (Figure 2).
Punch Biopsy. A punch biopsy instrument is inexpensive and versatile because it is available in a range of sizes. A punch biopsy may often be obtained with light sedation and a local anesthetic block, little suturing is required, and a larger tissue specimen is obtained than when a needle biopsy is performed. Punch biopsies are best taken from a dermal mass at the junction of normal and abnormal tissue. One limitation of this technique is the potential for inadequate sampling of subcutaneous or deep-seated tissues. A 3-mm or 6-mm diameter punch biopsy instrument is ideal for this technique (Figure 3).
Incisional Biopsy. An incisional biopsy may be preferred over a punch biopsy when a larger piece of tissue is needed or for sampling a subcutaneous tumor. This technique usually requires general anesthesia and is best performed using a scalpel blade. An incisional biopsy will also provide the pathologist with a specimen from which to make assessments of invasion and malignancy.
LESS-DESIRABLE BIOPSY TECHNIQUESFine-Needle Aspiration. Fine-needle aspiration may fail to yield a diagnosis, particularly if the tumor has necrotic areas or if there is a lot of blood in the aspirate. For this reason, other techniques are recommended to minimize delay in definitive treatment.
Excisional Biopsy. An excisional biopsy may compromise definitive treatment by altering tissue architecture and seeding surrounding unaffected tissues.
FURTHER STEPSAfter obtaining an incisional, punch, or needle-core biopsy, the clinician is advised to consult with an experienced surgeon and/or radiation oncologist. If aggressive surgery or radiation therapy is planned, further pretreatment staging should include CT scan or MRI to allow delineation of the margins of the tumor for appropriate surgical and/or radiation therapy planning (Figure 4).1
Treatment
SurgerySurgical excision has been the principal treatment method; however, due to the extensive infiltration and invasion of surrounding normal tissue, it is necessary to resect a wide and deep (more than 3 cm) margin of normal tissue in all surgical planes around the palpable tumor. The aim should be to remove the tissue en bloc without incising tumor tissue itself. While this is often possible in larger species, such as dogs and humans, it is more difficult in cats, particularly if the initial attempt was unsuccessful. Tumors on a distal extremity that can be amputated are an exception.
For this reason the first attempt at surgical removal should be the definitive one, and wide surgical margins that include bone, muscle, and other structures, if appropriate, should be obtained. For example, a cat with soft tissue sarcoma in the interscapular space would require resection that encompasses greater than 3 cm circumferential margins of normal tissue, including the musculature and dorsal processes of the scapulae and spine (Figure 5).
RadiationRecent studies have shown that radiation therapy is an excellent adjunct to surgery. Such therapy before surgical excision may have advantages over postsurgical radiation, but in either case survival times may be increased, and tumors are slower to recur.
The radiation field should encompass all tissue potentially infiltrated with tumor tissue. In order to limit the size of the radiation field and hence the risk for side effects, the definitive surgical procedure should be carefully planned.
Follow-up
PROGNOSISThe degree of histologic differentiation and mitotic rate found on biopsy of soft tissue sarcomas may be prognostic. In one study of 174 sarcomas, over half were characterized as fibrosarcomas, a third as pleomorphic sarcomas, 13% as spindle cell sarcomas, and 2% as undifferentiated sarcomas. Of eight cats that developed confirmed metastases, four had pleomorphic tumors, two had fibrosarcomas, and two had spindle cell sarcomas.
Mitotic Index. The mitotic index can vary widely in feline soft tissue sarcoma. In one study, the mitotic index of the tumor predicted recurrence and survival following surgery. Nineteen cats with a mitotic index of 4 or less had a median survival of 128 weeks. In contrast, 16 cats with a mitotic index of 5 or greater survived a median of 16 weeks.
Histology & Metastasis. Histologically, injection site sarcomas are locally invasive, have more mitotic figures, and appear more pleomorphic (less differentiated, more anaplastic) than noninjection site tumors. Injection site sarcomas may have increased risk for metastasis. In one study, staging procedures before initial treatment revealed no evidence of metastasis in any cat. However, after diagnosis and treatment, four cats developed metastatic disease from injection site sarcomas; only one cat developed metastatic disease from a noninjection site sarcoma.
BIOPSY OF SUSPECTED INJECTION-INDUCED SARCOMA IN CATS • Antony Stewart Moore
Reference1. Feline vaccine-associated sarcomas. McEntee MC, Page RL. J Vet Intern Med 15:176-182, 2001.
Suggested ReadingComparison of fibrosarcomas that developed at vaccination sites and non-vaccination sites in cats: 239 cases (1991-1992). Hendrick MJ, Shofer FS, Goldschmidt MH, et al. JAVMA 205:1425-1429,1994.Die fibrosarkome der katze unter besonderer berucksichtigung iher dignitat. Stiglmair-Herb VM, Ortmann U. Kleinteirpraxis 32:75-80,1986.Feline fibrosarcomas at vaccination sites and non-vaccination sites. Doddy FD, Glickman LT, et al. J Comp Pathol 114:165-174,1996.Feline Oncology: A Comprehensive Guide to Compassionate Care. Ogilvie GK, Moore AS - Trenton, NJ: Veterinary Learning Systems, 2001.Multicenter case-control study of risk factors associated with development of vaccine-associated sarcomas in cats. Kass PH, Spangler WL, Hendrick MJ, et al. JAVMA 223:1283-1292, 2003.Prognosis after surgical excision of fibrosarcomas in cats. Bostock DE, Dye MT. JAVMA 175:727-728,1979.
AcknowledgmentThe author thanks Dr. Greg Ogilvie for kindly providing some of the illustrations for this review.
Photo creditsAll figures reprinted from Feline Oncology. Ogilvie GK, Moore AS-Trenton NJ: Veterinary Learning Systems, 2001, with permission.