Antimicrobials are frequently prescribed for companion animals. Although mild adverse effects (eg, decreased appetite, nausea, other GI signs) are reported with many antimicrobials, alarming and severe adverse effects are possible—even with commonly used drugs.
Following are the top serious antimicrobial adverse effects, according to the authors, including prevention strategies to help ensure patient safety.
1. Retinal Degeneration in Cats Associated with Enrofloxacin
Enrofloxacin can cause acute blindness and retinal degeneration in cats.1,2 Defects in the ABCG2 gene that result in a dysfunctional drug transporter at the feline blood–retinal barrier are thought to contribute to accumulation of fluoroquinolones in the retina and resulting retinal degeneration.3 Most affected cats are administered enrofloxacin at >5 mg/kg in a 24-hour period, but blindness was reported in a cat given 4.6 mg/kg PO every 24 hours.1 Additional proposed risk factors include rapid IV infusions, increased patient age (>12 years), and renal impairment.2 Among reported cases in one study, affected cats were given enrofloxacin for a variable number of days (range, 4-73), and onset of blindness was detected by owners 2 days to 12 weeks after administration.1
Mydriasis is typically the initial sign of retinal degeneration, but fundic signs can include tapetal hyperreflectivity, retinal vessel attenuation, increased tapetal reflectivity, and optic nerve atrophy (Figures 1 and 2). Although some affected cats regain vision, others are permanently blind.

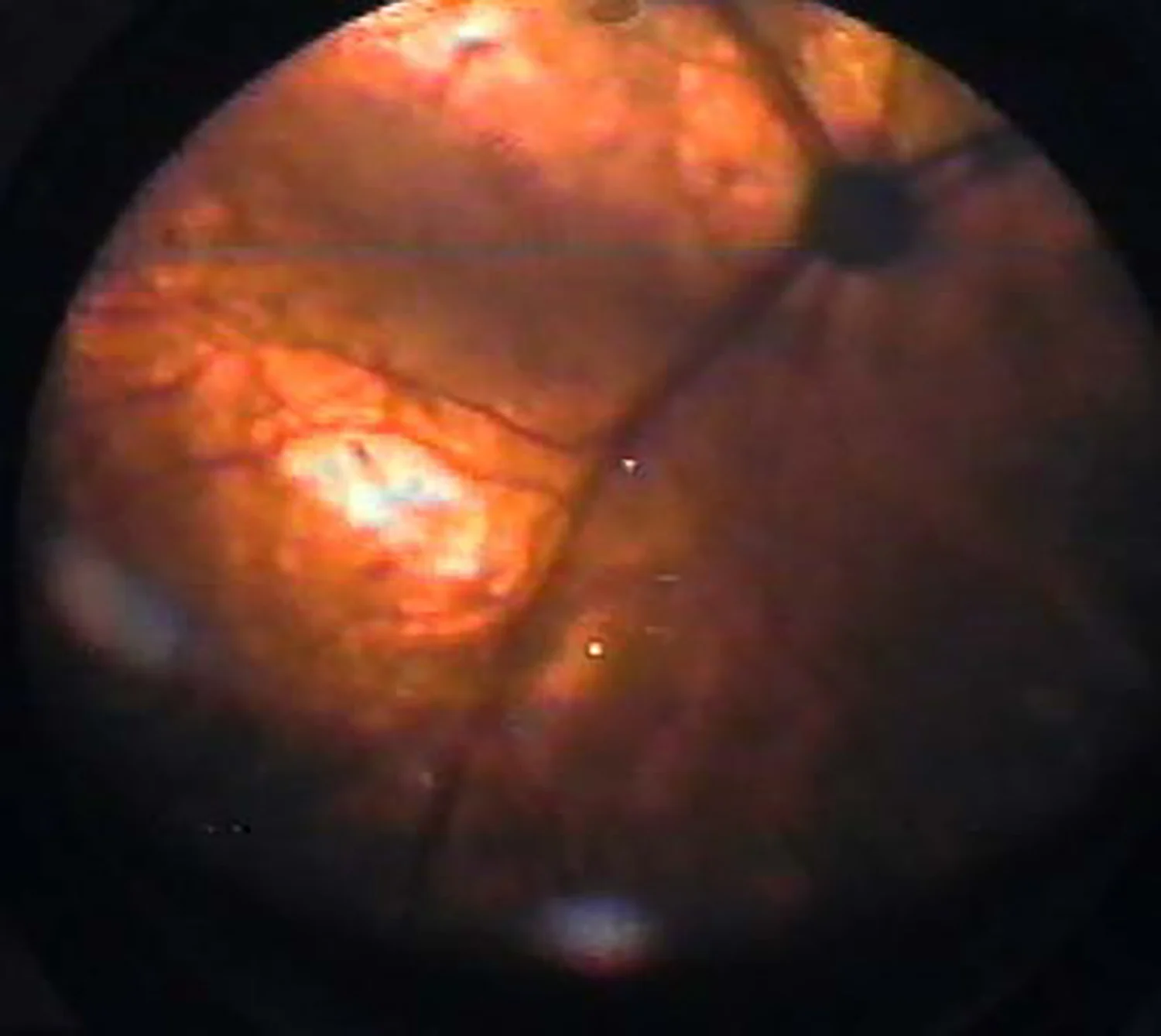
Retina of a 16-year-old cat with chronic kidney disease that became blind following enrofloxacin administration. The fundus shows tapetal hyperreflectivity (black arrow) and attenuation of retinal vessels (white arrow). Image courtesy of Anne Metzler, DVM, MS, DACVO

Fundus of a 3-year-old spayed Siamese cat with advanced retinal degeneration following enrofloxacin administration. Marked vascular attenuation (dashed arrow), increased tapetal reflectivity (solid black arrow), and optic nerve atrophy (white arrow) can be seen. The fundus is subalbinotic, which allows visualization of the choroidal vessels in the nontapetal portion of the fundus. Subalbinism is a normal variation in cats with blue irises. Image courtesy of Kansas State University Ophthalmology
Prevention of Retinal Degeneration Associated with Enrofloxacin
Fluoroquinolones should only be administered when culture and susceptibility testing for a confirmed infection suggest first-line antimicrobials are not appropriate. The labeled dosage of enrofloxacin (cats, 5 mg/kg PO every 24 hours) should not be exceeded; intravenous enrofloxacin should be avoided or diluted and administered slowly (injectable enrofloxacin is extra-label in cats); and treatment duration should be as short as possible. Other veterinary-approved fluoroquinolones may have lower risk for blindness, but caution should be used with all fluoroquinolones.2 A study in dogs demonstrated marbofloxacin does not accumulate in plasma of patients with renal failure.4 Although a similar study has not been performed in cats, marbofloxacin may be preferable to enrofloxacin in older cats and those with renal compromise, as the risk for retinal degeneration may be lower. Risks and benefits of fluoroquinolones should be explained to pet owners, and veterinary staff and pet owners should monitor the patient for mydriasis. Fluoroquinolone therapy should be immediately discontinued if mydriasis is noted during treatment, and a veterinary ophthalmologist should be consulted.
2. Immune-Mediated Disease in Dogs & Cats Associated with Potentiated Sulfonamides
Sulfonamides, including potentiated sulfonamides (eg, sulfadiazine, sulfamethoxazole, sulfadimethoxine with trimethoprim or ormetoprim), are commonly prescribed for coccidiosis and are recommended by the International Society for Companion Animal Infectious Diseases as first-tier options for bacterial cystitis; however, sulfonamides have increased risk for adverse effects compared with other first-tier antimicrobials (eg, amoxicillin, amoxicillin/clavulanate) for cystitis.5 The authors therefore prefer to reserve use of these drugs for antimicrobial-resistant cases indicated by culture. Standard doses can result in rare but severe idiosyncratic immune-mediated reactions in dogs due to T-cell–mediated delayed hypersensitivity.6 Dogs are at higher risk than cats, in part because dogs lack a metabolic pathway (ie, acetyl conjugation) to eliminate sulfonamides. Doberman pinschers, Samoyeds, and miniature schnauzers are predisposed, possibly because of alternate metabolic pathways that may lead to reactive and/or toxic metabolites.6,7
Clinical manifestations of immune-mediated disease include fever, polyarthropathy, acute hepatopathy, thrombocytopenia, neutropenia, hemolytic anemia, skin eruptions, keratoconjunctivitis sicca, uveitis, and even death.6-8 Ulcerative dermatitis lesions and mucocutaneous ulcers have been reported in cats.8 Survival rate of dogs with systemic signs is 77%.7 Thrombocytopenia and acute hepatopathy are negative prognostic indicators of survival.7 Immune-mediated reactions are typically seen 12 days (range, 5-36) after sulfonamide therapy is initiated.7
Prevention of Immune-Mediated Disease Associated with Potentiated Sulfonamides
Owners should monitor for inappetence, vomiting, diarrhea, lameness, lethargy, ocular discomfort, or darkened urine when administering sulfonamides or potentiated sulfonamides. If any of these signs are noted, therapy should be immediately discontinued, the patient should be examined, diagnostic testing (ie, CBC, serum chemistry profile, urinalysis, Schirmer tear test) should be performed, and prompt supportive care should be provided.
3. Esophageal Strictures in Cats Associated with Doxycycline & Clindamycin
Doxycycline and clindamycin have been associated with esophageal strictures in cats because these drugs have a low pH when dissolved.9,10 Solid dose drugs are easily retained in the midcervical esophagus of cats. Acidity from retained doxycycline or clindamycin capsules or tablets can cause esophagitis and esophageal strictures.11,12
Clinical signs (eg, regurgitation, hypersalivation, dysphagia, choking, gagging) of esophageal strictures can develop days to weeks following therapy initiation. Diagnosis is confirmed via esophagoscopy (Figure 3). Treatment typically consists of balloon dilation and supportive care (eg, sucralfate slurry, feeding tube) as needed.

FIGURE 3A
Esophageal stricture in a 2-year-old neutered male cat that developed within 2 days of treatment with doxycycline tablets for fever of unknown origin (A). Balloon catheter dilation using 6-, 8-, and 12-mm balloons sequentially was performed (B) to mechanically dilate the esophagus (C). Images courtesy of Ken Harkin, DVM, DACVIM (SAIM)
Prevention of Esophageal Strictures Associated with Doxycycline & Clindamycin
Doxycycline and clindamycin should be administered to cats as an oral suspension, and swallowing of capsules or tablets should be confirmed in dogs. If liquid is unavailable, tablets are preferred because they may carry less risk for esophageal damage than capsules; a bolus of water (eg, 2-6 mL) or food should be given immediately after the medication to speed transit of the antibiotic into the stomach.11-13 Doxycycline hyclate produces a more acidic solution than doxycycline monohydrate when dissolved in the neutral environment of the esophagus. Strictures are thus predominantly reported in cats given doxycycline hyclate, and doxycycline monohydrate is preferred if administration of capsules or tablets is necessary.10,14
4. Bone Marrow Suppression in Humans Associated with Chloramphenicol
Risk for aplastic anemia (very rare and idiosyncratic but typically fatal) and bone marrow suppression (dose-dependent and typically reversible) in humans are the most significant concerns when prescribing chloramphenicol for companion animals.15 Neither of these adverse effects are expected to occur from handling chloramphenicol (ie, transdermal exposure); however, chloramphenicol is on the National Institute for Occupational Safety and Health list of hazardous drugs, and precautions should be taken, including wearing gloves when handling chloramphenicol and avoiding crushing pills.16 Humans with underlying concerns (eg, bone marrow disease, chemotherapy) should avoid handling this medication.
Aplastic anemia from chloramphenicol does not occur in dogs or cats, but dose-dependent, reversible bone marrow suppression is possible and is seen more often in cats receiving >1 week of therapy.17,18 In cats, chloramphenicol can cause a mild to moderate decrease in packed-cell volume, neutrophils, lymphocytes, and platelets.17 In dogs, this effect is not likely to be clinically relevant, but chloramphenicol can cause mild suppression of erythropoiesis (as measured by packed-cell volume, hemoglobin, total RBCs) with no change in reticulocytes, megakaryocytes, or leukocytes.18
Prevention of Bone Marrow Suppression Associated with Chloramphenicol
Chloramphenicol should not be considered a first-line antibiotic and should be avoided in patients with underlying bone marrow disorders. Only short courses of therapy with dosages of 12.5 to 20 mg/kg (or 50 mg/cat) PO every 12 hours should be prescribed for cats, compared with 40 to 50 mg/kg PO every 8 hours for dogs.17,19 CBC should be considered every 1 to 2 weeks if therapy is prolonged, and owners should monitor for clinical signs of toxicosis (eg, decreased appetite, vomiting, diarrhea, depression).
5. CNS Toxicosis in Dogs Associated with Metronidazole
Metronidazole can cause dose-dependent CNS toxicosis (most often vestibular or cerebellar) in dogs.
Common signs of CNS toxicosis include vertical nystagmus, ataxia, inability to walk, upper motor neuron paresis, hypermetria, and vomiting related to vestibular disease.20,21 Signs can develop following administration of dosages previously recommended as safe (ie, 66 mg/kg PO every 24 hours; range, 33-110 mg/kg every 24 hours), with onset typically within 9 to 12 days after therapy is initiated.20 Most dogs fully recover following discontinuation of metronidazole and supportive care alone,20 but administration of diazepam (0.43 mg/kg IV, followed by 0.43 mg/kg PO every 8 hours for 3 days) can shorten recovery time from 11.6 days (range, 5-21) to 1.6 days (range, 1-3).21
Prevention of CNS Toxicosis Associated with Metronidazole
In dogs, metronidazole should be administered at ≤30 mg/kg PO in a 24-hour period for the shortest duration necessary to minimize risk for neurotoxicity.20 Twice daily administration of metronidazole has no known pharmacological benefit; once daily administration may thus be preferred for improved owner and patient compliance. For susceptible bacterial and protozoal infections in dogs, the authors routinely administer metronidazole at 20 mg/kg PO every 24 hours.22
Conclusion
Careful patient selection, drug formulation, dosages, and monitoring can help minimize risk for adverse effects, allowing safe and successful use of antimicrobials in companion animals.