Anesthesia Considerations in Small Mammals
Morgan Oakleaf, DVM, Colorado State University
Khursheed Mama, DVM, DACVAA, Colorado State University
Anesthesia is often required for procedures in small animals, but the risk for anesthesia-related death in small mammals is higher than in larger companion animals.1,2 Knowledge of anesthesia techniques and thorough preparation are essential to minimize complications.
Preanesthetic Evaluation & Preparation
Knowledge of effective restraint techniques designed to minimize stress while also preventing human injury (eg, bites) is important. For example, although gently grasping the scruff of the neck is effective in rats and mice, this technique may be less effective in a stressed hamster, which may still be able to turn its head to bite the handler. Rats may be briefly handled by the tail, which is not feasible for other small mammals. Iatrogenic injury from mishandling (eg, lack of support of the back and pelvic limbs of rabbits) is also of concern. Additional information on restraint and handling of small mammals can be found elsewhere (see Suggested Reading).
As in other species, a thorough history and physical examination are important. For example, knowledge of the patient’s diet and feeding frequency could provide information about nutritional status, and observation of discharge/discoloration on the antebrachium could suggest the patient is cleaning discharge from the face.
Additional information regarding diet and socialization or temperament may also be relevant. Obtaining an accurate weight is essential for appropriate drug administration; the authors recommend using a gram scale. Due to the unique anatomy and physiology of small animals, preoperative fasting times vary and are generally much shorter than in dogs and cats. For example, guinea pigs— which have limited reserves and a high metabolic rate—may be fasted for 2 hours or less; however, the pharyngeal pouches may need to be cleaned out prior to anesthesia. Rabbits do not vomit, but fasting up to 4 hours may help limit stomach volume and lower GI tract fermentation to enable easier breathing during anesthesia. Clinicians should be aware that prolonged fasting in small mammals can increase the risk for ileus and hypoglycemia.3,4 Ferrets may regurgitate during anesthesia, so longer fasting times of up to 6 hours in otherwise healthy animals are recommended.
Sedative & Anesthetic Drugs
In addition to obtaining an accurate weight, use of insulin and tuberculin syringes will help deliver drugs at appropriate doses. Dilution of certain drugs may be necessary. To achieve sedation appropriate for the patient, SC or IV premedication can be easily implemented and can reduce patient stress during subsequent manipulations, such as placement of an IV catheter (Figure 1) or monitoring equipment.
Although the drugs used for sedation in small mammals are similar to those used in other species, doses vary widely, as pharmacokinetic and pharmacodynamic information is limited and drug effects can vary further with sex and strain in some species. Sedative and tranquilizer drugs include α2 agonists (eg, xylazine, dexmedetomidine), acepromazine, and midazolam. Whereas a dog may receive 3-10 μg/kg dexmedetomidine IM in combination with an opioid, a healthy rabbit may require a significantly higher dose (eg, 40 µg/kg IM). Similarly, in small mammals such as rodents, chinchillas, and rabbits, doses for acepromazine (0.1-0.5 mg/kg IM) tend to be higher, but ferrets frequently respond to doses similar to those used in cats and dogs. Opioids are commonly combined with sedative and tranquilizing drugs to provide periprocedural analgesia; due to limited published information, clinicians often develop a drug preference based on their experience related to efficacy and adverse effects (eg, prevalence of ileus). Dose considerations are similar to those for tranquilizers (see Suggested Reading for specific dosing recommendations).
Adjunctive techniques such as CRIs for inhalant dose reduction and analgesia should be considered. The efficacy of systemic fentanyl and lidocaine administered via IV CRI has been demonstrated in rabbits.5-7 Regional analgesia techniques (eg, epidural administration of opioids or local anesthetics) may also be used in addition to administration of an NSAID (eg, meloxicam), which is typically done in the recovery period. Doses for these drugs are consistently higher than in cats and dogs.

Catheter in the lateral tail vein in a rat
Instrumentation Support & Monitoring
Venous access can be challenging and time- consuming but allows for delivery of analgesic, supportive, and emergency medications, fluids (5-10 mL/kg/hour in patients with normal packed cell volume [PCV] and total protein [TP]), electrolytes, and dextrose. Catheterization by skilled staff members, especially in critical patients, is generally advised. Small volumes (≤1 mL depending on the size of the animal to avoid fluid overload) of diluted heparinized saline or regular saline are recommended when flushing the catheter. All efforts should be made to avoid entry of even a small air bubble in venous circulation. Species-dependent sites for venous catheterization include the cephalic vein, medial or lateral saphenous vein, tail, and auricular veins. Anecdotally, in rabbits, sloughing may occur when auricular veins are traumatically catheterized or hyperosmolar solutions are administered through them. The size of the patient and location of the catheter will determine catheter size, which can range from 27- to 22-gauge for all patients, excluding the largest rabbits. Intraosseous cannulation of the humerus, ulna, tibia, or femur is occasionally used as an alternative but requires heavy sedation or anesthesia (see Suggested Reading). For minor procedures in which IV access is not needed, warmed SC fluids (10-20 mL/kg depending on the length of the procedure) may be administered.
Patient size, availability of appropriate equipment (eg, laryngoscope, endotracheal tube), and unique upper airway or oral anatomy (eg, pharyngeal pouches and palatal ostium in guinea pigs, strong jaw tone even at a good anesthetic plane in ferrets, caudally located larynx in rabbits) can contribute to intubation challenges in small mammals. Thus, many strategies—from blind intubation to use of a fiberoptic endoscope—have been advocated. A low volume (so fluid does not migrate down the airway) of topical lidocaine may be applied to help reduce laryngospasm. Alternative airway management strategies—including the laryngeal mask airway (available for rabbits and rats) or a fitted sealed face mask (Figure 2)—are often used and can facilitate ventilatory support in addition to delivery of oxygen and inhalant (see Suggested Reading). The system may also be adapted to provide heat.

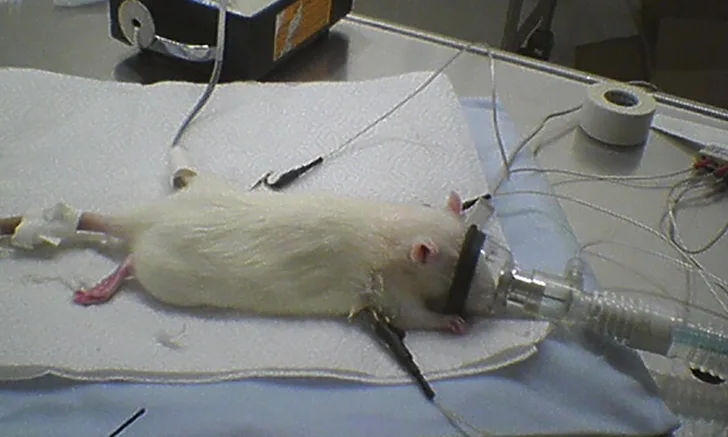
Rat with catheter, Doppler probe, ECG, and face mask
Because of their large surface area:body weight ratio, small mammals tend to lose body heat rapidly after receiving sedatives and anesthetics. To minimize energy use and ensure a timely recovery, external heat support should be maintained from premedication into recovery, and body temperature should be monitored and maintained at a range appropriate for thespecies. Heating pads, forced air units, heat lamps, and warmed breathing circuits may be used to facilitate patient warming. However, although small patients cool rapidly, it is also possible to overheat them, so vigilance is advised.
Placement of anesthetic monitoring devices following sedation is recommended. An electrocardiogram for monitoring of heart rate and rhythm should be connected using atraumatic clips. The clinician should recognize that monitors may not provide an accurate numerical value for heart rate unless designed specifically for small mammals. However, handheld multiparameter monitors, which are increasingly available, allow for straightforward continuous monitoring. A Doppler crystal allows for an audible pulse signal, and, in an appropriately sized patient, a cuff (sized to 40% of the extremity circumference) placed proximally to the crystal facilitates systolic blood pressure measurement (Figure 3). Oscillometric technology may be substituted if a cuff can be placed but without verified accuracy.
Pulse oximetry with a probe placed on the ear, tongue, or a limb is recommended to monitor oxygen saturation. A capnograph, although likely inaccurate relative to the arterial carbon dioxide value, can confirm a patent airway. A side stream capnography line attached to the endotracheal tube adaptor helps minimize dead space created by other side-stream adaptors and weight between the endotracheal tube and breathing circuit, as might occur with a mainstream capnograph. Capnography is important even in intubated patients because the tube size is small and can easily occlude with secretions.
Additional recommendations include preoxygenation prior to injectable anesthetic induction, fluid administration prior to and during the procedure with appropriate supplements (eg, dextrose) when warranted, eye lubrication and protection, and evaluation of the oropharynx for secretions prior to extubation.

Instrumented rabbit with blood pressure cuff proximal to Doppler crystal
Management of Complications
Anticholinergics may be used pre-emptively with some sedatives or tranquilizers and should be readily available with a dose predrawn in case of unexpected bradycardia during anesthetic management. Approximately 50% of rabbit species have atropine esterases that rapidly break down atropine, but atropine can still be administered in an emergency in these species and is useful due to its rapid onset.8 Glycopyrrolate may be used as an alternative, as it has a longer duration but a slower onset.
Ranges for normal blood pressure vary among small mammals (see Suggested Reading). Anesthetic hypotension may be observed in any species and is further influenced by sedative and anesthetic medications. Management includes decreasing the anesthetic dose and providing an appropriate fluid bolus. Studies of vasoactive drugs in rabbits have indicated that phenylephrine is more effective than the inotrope dopamine for maintenance of cardiac output.9 These and other emergency medications (eg, epinephrine) should be kept readily available to facilitate rapid intervention when needed. It is some clinicians’ preference to have these additional emergency medications drawn up along with reversal agents for sedative/anesthetic drugs when available. The dose of the reversal drug is generally proportional to the dose of the primary drug but may be reduced with duration from initial drug administration.
CASE EXAMPLES
Case 1
A healthy, 1-year-old, 4.6-lb (2.1-kg) intact male Angora rabbit was presented for routine castration. Both testicles were palpable on physical examination. Body temperature was 102.7°F (39.3°C), heart rate was 180 to 210 bpm, and respiratory rate was 60 to 80 breaths per minute.
Mucous membranes were pink and moist, and PCV and TP were 45% and 6.2 g/dL, respectively. The patient was not fasted prior to anesthesia.
The anesthesia plan included buprenorphine (0.03 mg/kg IM), dexmedetomidine (40 μg/kg IM), and ketamine (7 mg/kg IM). Oxygen and isoflurane were provided as needed via face mask. An appropriately sized laryngeal mask airway was available. Intratesticular block was achieved with lidocaine (2 mg/kg).
An ECG, pulse oximeter, Doppler probe (which usually provides a good signal from the brachial artery if placed over the medial aspect of the thoracic limb), rectal temperature probe, and adapted capnograph were implemented. Warmed isotonic crystalloid fluids (10 mL/kg SC) were administered. The eyes were lubricated and closed, and a warming forced air blanket was placed under the rabbit following drug administration.
The patient received meloxicam (1 mg/kg SC) postoperatively. Reversal of dexmedetomidine with a partial dose of IM atipamezole was considered if recovery was prolonged. An additional dose of buprenorphine was administered as needed for pain relief.
Case 2
A 2-year-old, 13.4-oz (380-g) intact female rat was presented for removal of an ≈2-cm round mass on the dorsal neck/shoulder area.
Temperature was 97.4°F (36.3°C), heart rate was ≈380 bpm, and respiratory rate was ≈100 breaths per minute. The pet owner reported that the patient was otherwise healthy and was a free-choice eater and not fasted. Blood work was declined.
The anesthesia plan included methadone (2 mg/kg IM), midazolam (2 mg/kg IM), ketamine (10 mg/kg IM), and glycopyrrolate (0.02 mg/kg IM) divided at 2 sites for sedation. Isoflurane to effect was provided via face mask to facilitate instrumentation.
The patient was placed on a forced air warming blanket from the time of sedation. An ECG was placed with atraumatic clips, and a Doppler crystal was placed on the patient’s tail. The patient’s eyes were lubricated prior to placement of the face mask to deliver isoflurane in oxygen. An adapted capnograph was used to monitor respiration. Intubation was not attempted. An IV catheter was placed for administration of fluids with 1% dextrose (10 mL/kg/hour) and in the event that blood loss warranted additional acute support.
During recovery, a bupivacaine block (2 mg/kg) was performed at the surgical site. Meloxicam (1 mg/kg SC) was administered if the patient was still considered to be in pain. Although pain can be challenging to assess in small mammals, facial grimace scales are now well-described in rodents. In addition, lack of grooming, hunched posture, abdominal contractions, and chromodacryorrhea (ie, red tears) may be used to assess this condition in rats.
Case 3
A 5-year-old, 3.7-lb (1.7-kg) intact female Holland Lop rabbit was presented for abdominal exploratory surgery and possible ovariohysterectomy; uterine adenocarcinoma was suspected based on history, physical examination, and abdominal ultrasound findings. Temperature was 101.2°F (38.4°C), heart rate was 280 bpm, and respiratory rate was 48 breaths per minute. Preoperative blood work was within normal range. The patient was resistant to palpation of a large abdominal mass and appeared anxious in the cage. Food was withdrawn from the cage 2 hours prior to anesthetic drug administration.
The anesthesia plan included hydromorphone (0.2 mg/kg IM), midazolam (0.5 mg/kg IM), ketamine (2 mg/kg IM), and glycopyrrolate (0.005 mg/kg IM) divided at 2 sites. Additional ketamine and midazolam were administered as needed for IV induction, and IV fluids were started prior to anesthesia and continued at 10 mL/kg/hour during the procedure. Anesthesia was maintained via sevoflurane administered to effect and lidocaine (100 μg/kg/minute). Fentanyl (20 μg/kg/hour) was considered in case this strategy was inadequate. In addition to use of a Doppler probe, an auricular arterial catheter was used for invasive blood pressure monitoring and blood sampling to monitor PCV and TP in case of hemorrhage. An incisional block using ropivacaine (1 mg/kg) was performed prior to surgical incision.
For recovery, administration of incisional ropivacaine (1 mg/kg) was repeated, and meloxicam (0.5 mg/kg SC) was administered. Reversal of midazolam with IV flumazenil was considered. Additional doses of hydromorphone were administered SC as required.