Examination of the Ear
Louis N. Gotthelf, DVM, Animal Hospital of Montgomery & Montgomery Pet Skin and Ear Clinic; Montgomery, Alabama
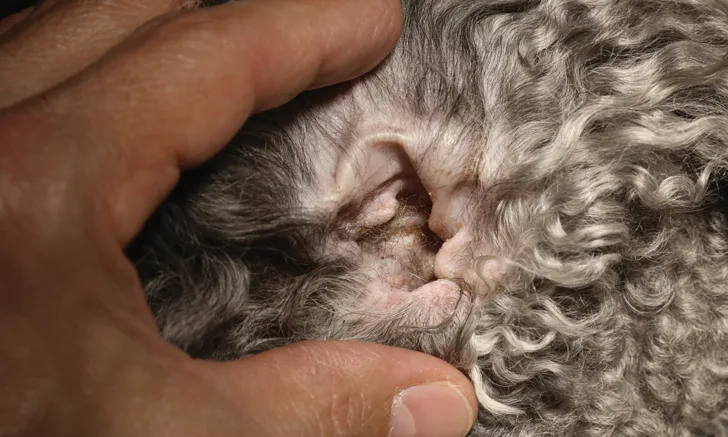
How should I conduct thorough ear examinations in my patients?
A thorough physical examination of the ear should include 3 independent parts:
Pinnal and preauricular skin examination
Otoscopic examination
Tympanic membrane assessment
Pinnal & Preauricular Skin Examination
Examination of the pinnae to assess for primary and secondary lesions can help formulate a list of differential diagnoses and diagnostic tests. Lesions found on the pinnae may also suggest conditions such as allergy, inflammatory/autoimmune conditions, infections, and infestations.
Causes of Pinnal Disease
Trauma
Hematoma
Ectoparasites
Immune-mediated disease
Keratinization defects
Malassezia spp and dermatophytes
Neoplasia
Atopic and contact dermatitis
Endocrine disorders
Pustules on the pinnae (Figure 1) can suggest either infectious (eg, pyoderma) or sterile disease (eg, pemphigus foliaceus). Cytologic examination of the pustules may show acantholytic cells and neutrophils indicative of pemphigus. Neutrophils and bacteria can be seen in some infectious causes of pustules. Papular eruptions may also be seen with parasitic diseases.

Pinnal erythema on the concave surface in an English bulldog with atopy. Note the presence of pustules and papules.
Thick scale and erythema of the pinnal margins, particularly the apices, may indicate sarcoptic mange, especially when a pinnal-pedal scratch reflex occurs in response to rubbing of the pinnae. In addition, erythema, crusts, and scale may be present with demodicosis or dermatophytosis. Low-power examination of skin scrapings placed in mineral oil on a microscope slide may help identify the presence of parasites. Fungal culture may be indicated.
If vasculitis is suspected as a result of ulceration, necrosis, notching, and/or scarring of the pinnal margin, a biopsy may be necessary to examine for capillary thrombosis. Ear margin seborrhea, a nonpruritic primary cornification defect often found in dachshunds and Yorkshire terriers, may result in keratinaceous debris or alopecia along the ear margins.1 Cytologic examination of secretions may disclose colonization by Malassezia spp.
A pinna that is no longer erect may indicate ear pain. Palpation of the pinnal cartilage, especially at the ear base, may indicate bony changes suggestive of chronicity. Aural hematoma typically indicates pain or pruritus in the ear and can result from violent head shaking, trauma, or autoimmune diseases.2 An aural hematoma on one side may result from otitis externa in the opposite ear.
Excoriation of the pinnal surface indicates self-trauma from pruritus that is often caused by otitis externa. Pinnal dermatitis and crusting with accumulation of purulent material on the base of the concave pinna may result from severe suppuration with otitis externa. Hemorrhagic crusting of the ear tips of erect-eared breeds and of the pinnal folding in pendulous-eared dogs may indicate fly strike or mosquito bite hypersensitivity in cats. Traumatic injury to the pinna may mimic other conditions.
Lichenification, erythema, hyperpigmentation, and glandular hyperplasia of the concave pinnae may indicate chronic allergic dermatitis, often with concurrent otitis externa. When found in cocker spaniels, this may be a manifestation of primary idiopathic seborrhea associated with ceruminous otitis.3 Topical drug reactionsparticularly to neomycin-containing otic medicationsmay also result in pinnal erythema and/or erosions or ulcerations.
Many pinnal tumors exist, including ceruminous gland adenomas in dogs and squamous cell carcinomas in white-eared cats.
Otoscopic Examination
In nonanesthetized patients, an otoscope cone of the proper diameter is gently inserted into the vertical ear canal. Once the ear canal lumen is centered in the eyepiece, the otoscope cone is advanced deeper into the ear canal. This technique avoids painful scraping of the sides of the ear canal.
As the canal narrows and bends, the pinna is grasped gently and moved outward and downward to straighten out the ear canal. After making the bend into the horizontal ear canal and advancing the speculum, the eardrum should be visualized (Figure 2).

Examination of the ear with an otoscope. The pinna is elevated to examine the vertical ear canal and is gently pulled down and out to examine the horizontal ear canal.
In nonclinical dogs without any apparent signs of otitis externa, only a quick otoscopic examination is necessary. After a veterinarian becomes familiar with the normal canine and feline ear, pathologic changes should be easy to identify.
Some patients — healthy or otherwise — will require sedation or anesthesia. Sedation is also recommended for any patient that is markedly painful and/or fails to respond to empiric therapy. This is especially important for patients with unilateral ear disease as a source of discomfort (eg, foreign body lodged in the ear canal).
The Ear Canal
The ear canal is lined by a modified epidermis. The wide vertical ear canal contains hairs surrounded by sebaceous glands, as well as aprocrine glands unassociated with hair follicles. The normal vertical ear canal glistens because of the cerumen coating (Figure 3). On examination of the ear canal with the otoscope, the skin of the vertical and horizontal ear canals should be nonerythematous. The epithelium of the ear canal is smooth. A network of blood vessels in the dermis is present.

Normal canine vertical canal. Blood vessels are visible in the dermis through the thin epithelium. A glistening coating of cerumen is present on the epithelial surface.
In most dog breeds, the narrower horizontal ear canal has significantly fewer hair follicles with minimal glands. The exceptions are the American cocker spaniel, which has more glandular tissue density along the ear canal than do many other breeds,4 and the standard poodle, which anecdotally seems to have a higher density of hair follicles along the ear canal.
Visual Examination of the Ear Canal
The canine ear canal consists of overlapping cartilage plates in a bent, funnel-shaped, tapered, cylindrical structure that provides the L shape to the vertical and horizontal ear canals. Cats have more of an arc shape to their ear canal than do dogs. Before inserting the otoscope, palpation of the vertical ear canal should be performed to assess cartilage plasticity. Any pain or bony changes to the cartilage should be noted.
Bacterial and yeast otitis externa are typically confined to the ear canal and/or pinnae. However, bacterial exudates from chronic otitis externa pooling in the horizontal canal can cause erosion of the eardrum leading to secondary otitis media or interna. With neoplasia, trauma, or severe otitis externa, extension of infection to surrounding tissues (cellulitis, abscess) is possible.
Cerumen
Cerumen is composed of apocrine (watery) and sebaceous (lipid) secretions as well as desquamated keratinocytes. Normal cerumen coats the epithelium, protects against pathogens, and aids in prevention of water loss from the ear canal epithelium. Its stickiness helps trap particles and other debris.
In human cerumen, antimicrobial lipids (eg, sapienic and lauric acids) and antimicrobial peptides can be beneficial against many fungi and bacteria. These substances have yet to be identified in canine or feline cerumen.5 Cerumen changes its composition in the presence of disease; it becomes less waxy and more hydrated. Normal cats have scant, thin cerumen but may produce excessive cerumen in response to diseases such as atopy or endocrine disease.
Removal of mild cerumen accumulations in normal ears using ear cleaners is not recommended. The normal process of epithelial migration removes accumulated ceruminous secretions as the surface epithelial cells move toward the outside of the ear canal to maintain ear health. In many ear diseases, this physiologic cleaning mechanism is disrupted and copious cerumen accumulates in the ear canal.
Hair Loss
Clinical signs of otitis with exudate in the ear canals may result in a depilatory effect from caustic enzymes dissolving the keratin that comprises the hairssimilar to the hair loss seen on the body in the form of an acute, moist dermatitis or hot spot. Clinically, this is identified as an absence of hair found in the ear canal during the acute phase of otitis externa. With resolution of ear disease, hairs will regrow as the epithelium returns to normal, and new hair growth can be seen on otoscopic examination at recheck.
Parasites
Live ear mites may be seen via otoscope crawling along the epithelial surface when a patients head is held as still as possible. Cats tend to have larger numbers of mites; the white-colored mites in the ear canal are seen crawling along the dark brown otic secretions. Other parasites (eg, ticks, flies) or foreign material (eg, grass awns, seeds) may be seen in the canal.
Stenosis & Swelling
Any change in the epidermis, such as with ceruminous gland hyperplasia or ulceration, is significant and should be noted (Figure 4). In a patient with inflammatory ear disease, thickening of the epithelium may obscure visualization of blood vessels. The clinician should assess for presence of stenosis. Some dog breeds (eg, shar-peis, pugs) have small-diameter ear canals as part of their standard conformation. Differentiation between swelling and stenosis is necessary. Stenosis results from permanent pathologic changes within the epidermis of the ear canal (Figure 5).

FIGURE 4
Cerumen gland hyperplasia. Inflammation in the ear canal results in increased secretions from the apocrine glands, which enlarge above the epithelial surface.
For swelling of the ear canal caused by inflammation, it may be necessary to delay a complete examination of the ear canal until anti-inflammatory therapy (eg, systemic or topical corticosteroids) decreases the swelling.
If changes do not reverse, the prognosis for medical management is poor, and total ear canal ablation and/or bulla osteotomy is recommended. If the ear canal epithelium is smooth and pink in color but the ear canal diameter is narrowed, hyperplasia of the sebaceous glands may be present.
Ulceration
Ulceration of the ear canal epithelium is most often associated with a severe, chronic bacterial otitis externa (often Pseudomonas spp). The bacteria releases cytopathic enzymes resulting in ulceration (Figure 6). Tumors within the ear canal may be present and should be noted (Figure 7).

FIGURE 6
Ulceration of the ear canal. Note the disruption of the surface epithelium.
Exudates
Note the character and volume of exudates. In general, a presumptive diagnosis of the infection type can be made based on texture and color. Dark brown waxy or dry exudates suggest yeast otitis in dogs or ear mite infestation in cats (Figure 8). In dogs, creamy, tan-colored moist exudates tend to suggest gram-positive (eg, Staphylococcus spp) bacterial infections (Figure 9).
Purulent liquid or mucoid exudates with a white, yellow, greenish, or black color may indicate a gram-negative (eg, Pseudomonas spp) bacterial infection (Figure 10). Hemorrhagic exudates can indicate ulceration of the ear canal or ulceration of a tumor mass. An ear canal epithelial examination may not be possible until the exudates are flushed out of the ear canal and the epithelial surface is exposed.
Mucus should never be found in the external ear canal; there are no goblet cells in the epithelium. Goblet cells abound in the lining of the middle ear, which is a mucous membrane. Excessive amounts of mucus can be found in the middle ear when it is inflamed and may move from the middle ear to the external ear canal through a hole in the eardrum.
All otic exudates should be sampled for cytologic examination to characterize the type of organisms present (ie, cocci, rods, yeast). An erythemic and exudative ear canal may often result in pruritus and pain without infection. This would support inflammatory disease of a primary nature (eg, allergy).
Additional Considerations
Bacterial culture and antibiotic susceptibility testing may be helpful to determine appropriate treatment, especially in refractory cases. Bacterial culture rarely reports growth of Malassezia spp yeasts; therefore, sending a culture off without cytology may result in information that has no bearing on the otitis case being treated. Some bacteria cultured from the ear may not be the pathogen(s) perpetuating the ear disease.
There is significant variability in antibiotic susceptibility testing among reference laboratories.6 Susceptibility testing may be used as a guideline in selecting topical ear therapy. The authors preferred tools for determining whether an antibiotic is helping include follow-up ear examinations to assess clinical improvement and comparative cytologies to determine the presence, reduction, or absence of microorganisms from previous examinations. Resistance to an antibiotic on susceptibility testing may be misleading when treating otitis externa, as these susceptibilities are reported for blood levels. Topical antimicrobial levels may be hundreds of times more concentrated in the ear canal and may be effective in the face of reported in vitro resistance.
Ceruminoliths, concretions of dried medications, and foreign bodies are more often seen in the horizontal canal. Occasionally, epilated hairs will fall into the horizontal canal and become embedded in the thick wax accumulation along the floor of the horizontal canal. These hairs can be seen occluding the view of the eardrum (Figure 11).

FIGURE 11
Hairs in the horizontal ear canal obstructing the view of the eardrum
A feline inflammatory polyp originating from the middle ear may also be seen as a fleshy mass protruding into the horizontal canal (Figure 12).
Tympanic Membrane Assessment
Examination of the tympanic membrane (ie, eardrum) is crucial and can be performed in almost all healthy patients without sedation.
The eardrum has 2 parts:
Pars flaccida: an upper, fleshy, vascular portion
Pars tensa: a thin, translucent portion
The pars tensa transmits sound vibrations through the bones of the middle ear to the cochlea. Embedded in the pars tensa is the manubrium (handle) of the malleus bone. In dogs, there is a gentle hook to the malleus that points rostrally; in cats, this structure is thinner and straight. The pars flaccida provides the blood vessels that feed the pars tensa (Figure 13).
The eardrum must be examined to determine if it is normal or damaged or if purulent, ceruminous, or keratinaceous exudates are present in the bulla behind it.

Normal left eardrum in a dog. Note the tip of the malleus bone points rostrally.
An area of germinal epithelial cells several cell layers thick covers the malleus bone along the pars tensa. The epithelial cells of this germinal center replace themselves regularly, and the older cells move off the eardrum toward the horizontal ear canal, carrying any debris present on these cells. This keeps the eardrum pliable and relatively free of cerumen accumulation.
Damage to the tympanic membrane caused by trauma (eg, chewing activity of ear mites, previously ruptured eardrum that has healed) or chronic infection disturbs this epithelial migration process. When epithelial migration is slowed, as with age or from disease, debris, cerumen, and hairs accumulate on the eardrum. A ceruminolith may develop on the eardrum on the external ear canal side (Figure 14). These plugs may also consist of dried mucus or medication that has hardened in the horizontal canal.

Wax plug (ceruminolith) on the eardrum
Cerumen accumulation against the eardrum, in addition to stenosis and hyperplasia, can impede conduction of sound waves and result in temporary hearing loss. Ceruminoliths require mechanic removal, preferably with endoscopic grasping forceps through a video otoscope with the patient anesthetized. If the eardrum is not intact, exudates such as mucus and pus can leak into the horizontal canal and inspissate at the eardrum. This condition can look like a ceruminolith. The mucoid secretions dissolve in water and can be removed with a water or saline flush of the horizontal canal.
Because the normal pars tensa is translucent, the bulla cavity can be seen when enough light (using a video otoscope) transilluminates the bulla. In otitis media, fluid, if present, can be seen behind the eardrum in the form of bubbles, blood, serum, or pus. Increasing pressure from exudate accumulation can cause the pars tensa to bulge outward. Both of these conditions result in marked pain; pressure must be relieved by myringotomy. Any tissue mass in the middle ear will obscure the ability to view the middle ear (Figure 15). Occasionally, in cats, a middle ear polyp can be seen through the eardrum as a fleshy mass in the bulla.

Tissue mass behind the eardrum. Note the malleus bone in the pars tensa, indicating the mass is behind the eardrum.
The eardrum may be opaque from chronic inflammation and thickening and/or hyperkeratosis. If the eardrum is opaque, the middle ear cannot be evaluated visually from the ear canal.
In some dogs, the pars flaccida of the eardrum may be bulging from fluid pressure in the middle ear behind it (Figure 16) and may obscure visualization of the pars tensa. Some have suggested that this enlargement may be caused by degranulation of mast cells and resultant edema from atopic dermatitis.7,8 This condition is frequently mistaken for a mass in the external canal and should be closely evaluated.

Bulging eardrum caused by increased fluid pressure in the middle ear
In the Cavalier King Charles spaniel, a condition called primary secretory otitis media can cause bulging of the eardrum resulting from the failure of mucoid drainage from the tympanic bulla through the auditory tube.
Cholesteatoma may be seen as a fleshy sac (epidermal cyst) at the level of the eardrum associated with the invagination of squamous epithelium into the middle ear. In longstanding cases of otitis externa with otitis media, the eardrum may not be present at all; a direct communication of the external ear canal to the middle ear cavity may be noted.