10 Dermatoses to Consider in the Young Patient
Karen Helton Rhodes, DVM, DACVD, Ceffyl Consulting, Canine Skin Solutions, Veterinary Council for Breed Stewardship
A number of dermatoses come to mind when examining a young patient. Most clinicians are familiar with more common presentations such as demodicosis, dermatophytosis, and superficial bacterial infections, but the following highlighted disorders may not be foremost in mind when formulating a list of differentials.
Sounding Board
A Letter to the Editor: Silver Coat Color in Labrador Retrievers & Misconceptions About Acids
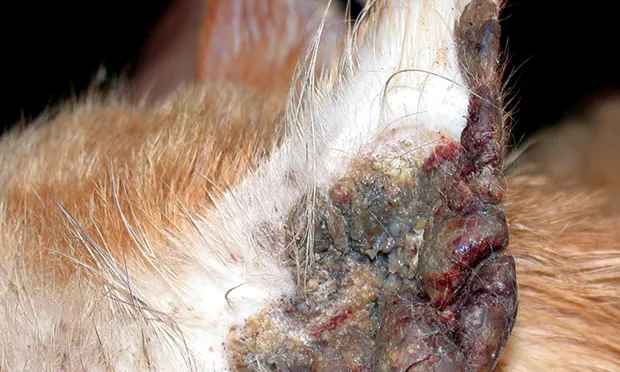
1. Feline Proliferative and Necrotizing Otitis
Feline proliferative and necrotizing otitis (Figures 1**-3**) is a visually distinctive syndrome of unknown cause. Patients initially recognized with this disorder were young kittens, but the age of onset now appears to be anywhere from 2 months to 12 years of age (mean, 4 years).1

Figure 1
Proliferative and necrotizing otitis externa in cats. Note the massive tissue proliferation causing stenosis of the canal
Cats typically present with acute, rapid, and severe proliferation of the vertical otic canal, external orifice, and concave pinna. The tissue is characterized as tan-to-brown coalescing, friable plaques. Handling of the tissue may cause ulcerations and hemorrhage.
Often, thick keratinous debris is present within the stenotic canal. The lesions are quite dramatic and may be bilateral. Some cats appear painful, whereas others may be pruritic or without clinical signs. Some patients will exhibit a secondary bacterial or Malassezia spp overgrowth.
Affected cats are otherwise healthy. Spontaneous regression of lesions has been reported.2 The exact cause of the disorder is unknown. On histopathologic examination, the tissue appears similar to hyperkeratotic erythema multiforme, another immune-mediated disorder.<sup3 sup>
No infectious causative agents have been identified as possible triggers. Anecdotal reports of success with topical tacrolimus, topical imiquimod, oral cyclosporine, famciclovir, and interferon are present in the literature.2 Debulking of the proliferative tissue with a CO2 laser may also be a consideration, but no data are available to support evidence-based therapeutic recommendations.
2. Eosinophilic Dermatitis and Edema (Wells Syndrome–like Disorder)
In humans, Wells syndrome is characterized as an eosinophilic cellulitis that exhibits similarities to eosinophilic dermatitis and edema seen in young dogs (Figure 4); this nomenclature is often used in the veterinary literature and dermatology lectures. The exact cause of eosinophilic dermatitis and edema is unknown, but adverse drug reaction is highly suspected.4

Severe eosinophilic dermatitis and edema in a dog. Note the serpiginous tracking and coalescing pattern of erythema in this patient.
A common immune trigger (eg, diet, arthropod vector, immune-mediated skin disease, drug, viral) has been speculated as causative. Most affected are large-breed dogs; 29% of reported cases are Labrador retrievers. Age of onset is typically 1 to 2 years of age.4
Approximately 60% of affected patients had a recent (<10-day) GI event with varying degrees of vomiting and diarrhea5; some dogs required hospitalization. Around 17% of reported cases had a concurrent onset of GI signs and cutaneous changes.5 Some patients had potential evidence supporting adverse drug reaction as a cause.
Dermatologic findings are unique and can help narrow the differential list. Lesions are characterized by erythematous macules that become arciform with serpiginous wheals and plaques. The ventral abdomen, thorax, and pinnae are the primary sites. Facial edema and generalized pitting edema may be seen in severe cases.
In the author’s experience, cutaneous histopathology may show an eosinophilic superficial and deep perivascular interstitial dermatitis with edema and vascular dilatation and prominent flame figures. Patients tend to respond rapidly and favorably to systemic corticosteroids. Some cases may wax and wane, yet most do not recur.
3. Acral Mutilation and Analgesia Syndrome
Acral mutilation syndrome (Figures 5-6) is an unusual hereditary (autosomal recessive) sensory neuropathy. Shorthaired pointers, English pointers, French spaniels, English springers, and miniature pinschers are primarily affected.6 Puppies typically start to show abnormalities by 3 to 5 months of age; initial signs include intense licking, biting, and chewing of the paws, with eventual mutilation and even autoamputation of the paws. Complete acral analgesia is often seen.

Acral mutilation and analgesia syndrome (originally described in German shorthaired pointer puppies)
Affected dogs walk with no demonstration of pain or discomfort. Motor abilities, reflexes, and proprioception appear normal. Diagnosis is made via clinical presentation and histopathologic examination of nerve tissue at necropsy. There is no known effective therapy for these patients. Cases are managed with oral gabapentin or amitriptyline, boots, bandages with ointments to prevent osteomyelitis, and an E-collar to help prevent self-trauma.
Humane euthanasia is often chosen based on the severity of signs. Parents and siblings of affected puppies should not be used in further breeding programs.


Acral mutilation syndrome
4. Ulcerative Nasal Dermatitis of Bengal Cats
This disorder (Figure 7) appears to be limited to the Bengal cat, a breed that is becoming more popular in the United States.7 There is no obvious cause, but breed limitation suggests that genetic factors might play a role.
The disease is not typically severe but is often cause for owner concern. The onset of clinical signs is between 4 to 12 months of age with no sex predilection noted.8 The nonhaired portion of the nose (nasal planum) becomes dry and scaly with progressive hyperkeratosis, fissuring, and crusting. Depigmentation can be seen. Topical tacrolimus rapidly resolves the nasal lesions.

Ulcerative nasal dermatitis of a Bengal cat. Note the early hyperkeratosis and fissuring
5. Juvenile Cellulitis
The argument can be made that this is not a rare disease but is frequently misdiagnosed. The disease goes by many names: puppy strangles, juvenile pyoderma, juvenile sterile granulomatous dermatitis, and lymphadenitis, among others. This nomenclature may be misleading; the disease is not a primary bacterial infection.
This disease is a sterile idiopathic granulomatous and pustular disorder, primarily affecting the face, feet, pinnae, and submandibular lymph nodes (Figures 8-10). The exact cause and pathogenesis of juvenile cellulitis is unknown. Heritability has been explored because of an increased incidence in certain breeds.2 Immunologic trigger factors likely play a large role, yet no specific factors have been identified.

Figure 8A
Juvenile cellulitis. Labrador retriever puppy with acute onset of muzzle and eyelid edema
Typical age of onset is 3 weeks to 4 months, but adult onset has been reported.2 Several breeds (eg, golden retrievers, Labrador retrievers, dachshunds, Gordon setters) appear to be predisposed. Initial clinical signs include swelling of the eyelids and muzzle. There is a rapid progression to swelling of the lymph nodes, papules, pustules, exudative otitis externa, and fistulous tracts around the head and neck region. Some puppies develop concurrent sterile pyogranulomatous panniculitis.
Bacterial overgrowth is typically secondary, but the lesions may clinically appear infectious. A thorough history can help differentiate this disease. Cytology and cultures from patients with juvenile cellulitis are negative for bacteria and will demonstrate a poor response to systemic antibiotics. Patients with angioedema do not have as much of a dramatic regional lymphadenopathy when compared to patients with juvenile cellulitis.
Juvenile cellulitis patients tend to exhibit malaise and pain on palpation of the affected regions; occasionally, fever is present.2 Histopathologic examination can confirm multiple or confluent granulomas consisting of clusters of large epithelioid macrophages and neutrophils.2 Suppurative changes in and around ruptured hair follicles with panniculitis is frequent in later lesions.
Therapy should be rapid and aggressive to prevent scarring. Systemic corticosteroids (2.2 mg/kg/day for 2 to 3 weeks with slow taper) are the therapeutic gold standard. Patients respond rapidly and favorably. Some older patients may require oral cyclosporine.
6. Hair Coat Color Disorders (Select Follicular Dysplasia)
This group of disorders, characterized as follicular dysplasias, tends to have a genetic or hereditary determination. Not all follicular dysplasias, however, are associated with coat color changes.
Affected dogs tend to exhibit an abnormal coat color as well as develop progressive alopecia at approximately 1 year of age; an increased incidence of superficial folliculitis has also been seen.
Color Dilution Alopecia (Color Mutant Alopecia)
The most recognized disorder in this category, this has been described in a number of breeds: dachshund, Great Dane, whippet, Italian greyhound, chow chow, Yorkshire terrier, silky terrier, Chihuahua, Boston terrier, Saluki, Newfoundland, Shetland sheepdog, German shepherd, schipperke, schnauzer, and the Labrador retriever, including the silver Labrador (Figure 11).2 The silver Labrador retriever is the newest breed to promote the silver color. A tentative diagnosis can be reached in-house with identification of macromelanosomes, or large clumped melanin granules in the hair shafts via trichography (ie, plucking hairs with a hemostat, then placing in mineral oil for microscopy). Cuticular defects can be seen at the site of melanin clumping along the hair shaft.2 A definitive diagnosis is made by histopathology demonstrating follicular dysplasia.

Figure 11A
Color dilution alopecia in a silver Labrador retriever. Note the thin hair coat with a diffuse light scale.
Color Dilution of the Rhodesian Ridgeback
The Rhodesian ridgeback has a unique form of color dilution. This breed has an autosomal recessive disorder that codes for a diluted coat color (blue) and neurologic abnormalities recognized at 2 weeks of age.9
Black Hair Follicular Dysplasia
This can be seen in both crossbreed and purebred dogs. Puppies are born with a lusterless hair coat and exhibit progressive and complete loss of the entire black-colored coat by 6 to 9 months of age.10
Follicular Lipidosis in the Rottweiler
A variant of follicular dysplasia, this is characterized by a complete loss of the mahogany points of the face and feet. Clinical signs typically start within the first 9 months of age.11 Other mahogany points and the black hair coat remain normal. Alopecia may resolve spontaneously or persist.
7. Viral Papillomatosis
Viral papillomas are not a new entity in clinical practice, but in the author’s experience there has been an apparent resurgence of young dogs presenting with canine oral papillomas (Figure 12). Young patients may present with multiple warts in the oral cavity, tongue, eyelids, lips, and skin. In the author’s clinical experience, this may be associated with increasing contact between young dogs in a variety of high-exposure situations (eg, day care, group dog-walkers, dog parks).


Mucocutaneous viral papillomatosis
There are a number of different clinical presentations of viral papillomatosis (see Signs of Viral Papillomatosis). Incubation period is typically 1 to 2 months after exposure. Papillomaviruses can survive in the environment for 63 days at 4°C to 8°C (39.2°F-46.4°F) or for 6 hours at 37°C (98.6°F). Clinical diagnosis is confirmed via histopathology.
Signs of Viral Papillomatosis
Humoral immunity protects against viral challenge, but it does not play a role in the clearance of established lesions. Cellular immunity is important for viral clearance, so vaccines are of limited help in the resolution of active lesions; however, they may aid in prevention.12-13 Likewise, recovering dogs are typically immune to reinfection.
A number of therapeutic options are available, yet supporting data are limited. Azithromycin, cimetidine, interferon alfa (oral or injectable), cryotherapy, surgical excision, CO2 laser ablation, crushing of lesions for antigenic stimulation, imiquimod, and recombinant CPV1 vaccine have been evaluated. Based on the author’s clinical experience, it appears that oral interferon at 10000 to 20000 U daily is often the most successful therapeutic value. There is a lack of evidence-based therapeutic data.
8. Familial Canine Dermatomyositis
Dermatomyositis (Figures 13-14) is a genetically determined (autosomal-dominant) immune-mediated disease, primarily affecting the collie and sheltie breeds, as well as Beaucerons.14
The age of onset can be as early as 7 weeks; affected animals usually develop signs before 6 months of age.15 The actual severity of the disease and progression of the lesions varies with each dog. Mild, nonscarring alopecia can be the only feature.


Familial canine dermatomyositis. Note the scarring alopecia over the facial region, which is typically one of the earliest affected regions.
Severe cases may involve alopecia, erythema, scaling, crusting, rare ulcerations, and residual scarring alopecia. Cutaneous lesions—most pronounced on the muzzle, pinnae, periocular region, digits, and tip of tail—may become generalized over time. Many affected dogs have abnormal nail morphology (eg, onychorrhexis, onychomadesis, onychoschizia).
Areas of mechanical trauma are often the most severely affected. Rarely, oral lesions are present. Myositis tends to follow the cutaneous changes and involves occipital muscle atrophy, atrophy of the muscles of mastication and distal limbs, megaesophagus, severe ligamentous laxity, and a high-stepping, stiff gait.

Familial canine dermatomyositis. The entire ventrum of this patient is scarred with serpiginous areas of postinflammatory pigmentation.
Cutaneous histopathology will show hydropic degeneration of the basal cell layer with apoptosis, pigmentary incontinence, and follicular atrophy.16 Muscle fibers exhibit necrosis and atrophy.
Patients should avoid trauma and sun exposure, as both may worsen lesions. Therapeutic options include vitamin E, essential fatty acid supplementation, pentoxifylline, and doxycycline with niacinamide. Signs may be controlled, but there is no cure.
9. Hereditary Cornification Disorders
The epidermal barrier and its role in a number of diseases (eg, ichthyosis, seborrhea, allergy) has become a subject of intense discussion and research. There is an alteration in the epidermal lipid barrier of humans with atopic dermatitis; this has recently been shown in atopic dogs as well.17 The exact defect has not been characterized, but focus on repairing the barrier holds some promise for effective therapeutic management of pruritic dermatitis.
The epidermal barrier (stratum corneum) controls hydration, is the primary defense against environmental hazards (eg, allergens, pollutants, irritants) by continuous desquamation, maintains homeostasis with commensal organisms via antimicrobial peptides (eg, defensins, cathelicidins), and absorbs ultraviolet light, which protects sensitive underlying tissue.
Hereditary cornification disorders disrupt the function of the epidermal barrier and have no cure; however, management protocols are available. Two cornification disorders have been described in 2 popular dog breeds. The first is an autosomal-recessive PNPLA 1 mutation in golden retrievers (Figure 15) that results in congenital ichthyosis. This gene plays a role in lipid metabolism and organization. Affected dogs exhibit adherent, large, white, flaky scales, typically by 1 year of age. A genetic test is available for detection of carrier status among breeding dogs.


Ichthyosis in a golden retriever. Note the poor hair coat (A) and the adherent, large, white, flaky scales (B)
The second disorder is a mutation in the NIPAL 4 gene in American bulldogs.18 Affected bulldogs have a decreased expression of the protein ichthyin, which plays a role in lipid metabolism in the epidermis.18 A genetic test is available. Affected bulldog puppies exhibit early clinical signs, often at weaning. They have a scruffy, disheveled coat compared with littermates. A light-brown adherent scale of the glabrous skin will be present. The scaling progresses to encompass the entire body. These puppies frequently develop secondary Malassezia spp overgrowth with subsequent pruritus and may be clinically mistaken as allergic patients.
Management
Efforts to repair the barrier are the therapeutic focus and can include 1 or more of the following: oral essential fatty acid supplementation, spot-on products with essential fatty acids or ceramides, antimicrobial shampoos with ceramides or emollients, rinses, lotions, and oral cyclosporine.
10. Fungal Kerion
This diagnosis is frequently overlooked and should be suspected in Persian cats or young dogs (<1 year of age) with small, non-healing dermal or subcutaneous nodules of the head or extremities.19 Diagnosis is obtained when these lesions are excised and submitted for histopathology. In the author’s opinion, it is likely that the lesions (Figure 16) represent dermatophytes that have become embedded in the deeper, non-keratinized tissue secondary to a ruptured hair follicle.
Excision is the best treatment for these lesions. Oral and topical medications are ineffective.

Fungal kerion. A solitary dermal/subcutaneous nodule.
CPV1 = canine parvovirus type 1