Pain & Reluctance to Move
Jennifer S. Thomas, DVM, PhD, Diplomate ACVP, Michigan State University
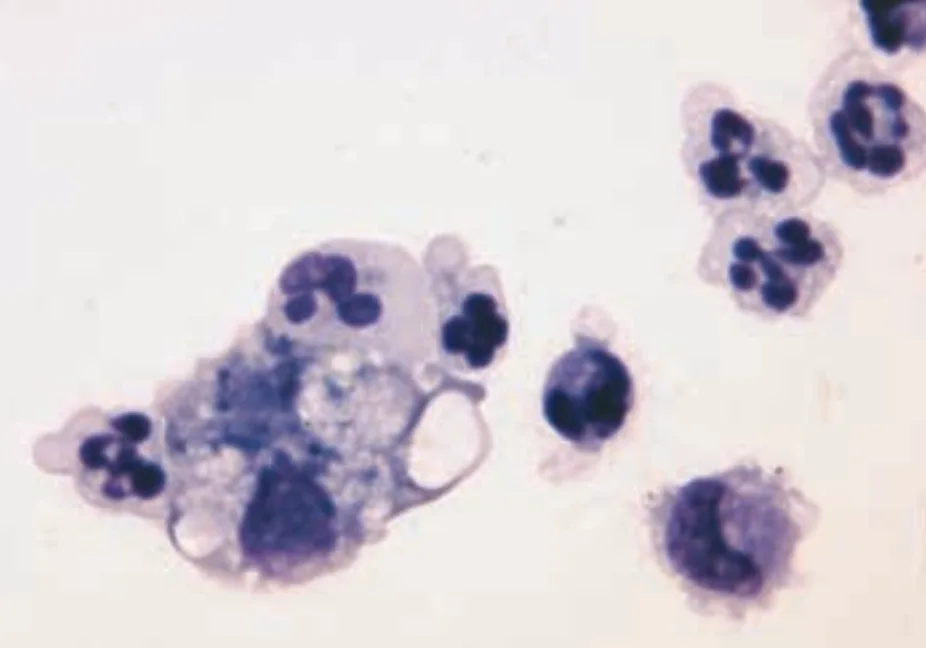
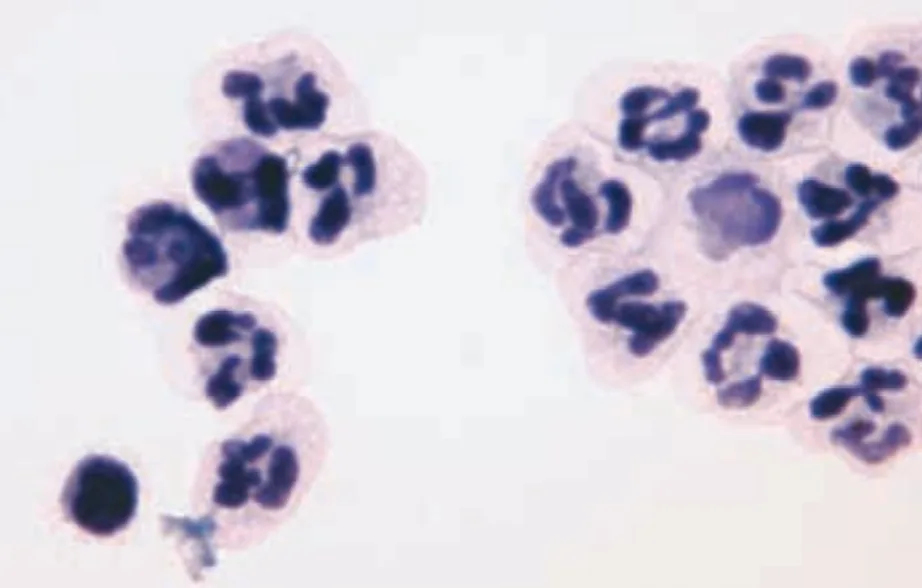
FIGURE 1A Cytospin concentrated smear of CSF. Analysis revealed nucleated cell concentration, 684/µl; RBC concentration, 5/µl; and microprotein concentration 91 mg/dl (modified Wright’s stain; original magnification, 100×).
FIGURE 1B Cytospin concentrated smear of CSF (modified Wright’s stain; original magnification, 100×).
CSF = cerebrospinal fluid; RBC = red blood cell
A 1-year-old male boxer was presented with a 1-month history of waxing and waning pain and reluctance to move. No response was noted after doxycycline administration. Physical examination revealed fever (temperature, 103.1º F), cervical rigidity, and pain on manipulation of the neck. Cerebrospinal fluid was collected, and cytospin concentrated smears were prepared.
Ask Yourself ...
What would you expect to find on cytologic examination of CSF from a healthy dog?
What microscopic findings are present in the smear from this dog?
What disorders are associated with these cytologic findings?
Diagnosis
Steroid-responsive meningitis-arteritis
Cytologic Evaluation. Cytospin concentrated smears of CSF contained large numbers of inflammatory cells and rare erythrocytes. The inflammatory cells were primarily nondegenerate neutrophils, many of which appeared hypersegmented. There were small numbers of lymphocytes, macrophages, and monocytoid cells. No erythrocytes or etiologic agents were present. The findings were consistent with neutrophilic pleocytosis.
Pathophysiology. Steroid-responsive meningitis-arteritis (SRMA) is the most common cause of meningitis with neutrophilic pleocytosis in dogs.1 It occurs most frequently in young-adult, medium- to large-breed dogs. Boxers, beagles, and Bernese mountain dogs are predisposed. Affected dogs have increased immunoglobulin A in the CSF and in the serum, suggesting an immune-mediated cause.1,2 Activated lymphocytes are present in some dogs, suggesting that SRMA may be initiated by exposure to exogenous (infectious or noninfectious) or endogenous antigens.1,3 Histologic examination reveals inflammation of the meninges and meningeal arteries. CSF culture is negative for bacteria. Long-term glucocorticoid therapy is recommended to resolve clinical signs.1,2
Clinical Signs. Fever, depression, cervical rigidity and pain, reluctance to move, stiff gait, and anorexia in the acute form. Ataxia, tetraparesis or paraparesis, and pacing in the protracted form.1,2
Diagnosis. Cytologic evaluation of CSF is an important diagnostic tool in the evaluation of patients with central nervous system disorders. Elevations in nucleated cell concentration and/or microprotein occur secondary to inflammation, infection, trauma, neoplasia, or degeneration of the brain and spinal cord.
The CSF is generally collected from the cerebellomedullary cistern if clinical signs suggest lesions in the craniocervical spinal cord or brain and from the lumbar subarachnoid space if signs suggest lesions in more caudal regions of the spinal cord.4 Collection from the lumbar space is more challenging, and the fluid is more likely to be contaminated with blood.5 CSF should be collected in a sterile plain tube for culture and cytologic evaluation. In cases of significant blood contamination or elevated nucleated cell counts or if fibrinogen is present, adding EDTA is recommended for cell counting.5
Cells break down rapidly in the low-protein environment of normal CSF. Cell counts and concentrated smears should be obtained within 30 to 60 minutes of collection. Concentrated smears should be prepared by using cytocentrifugation or sedimentation.6 Alternatively, an aliquot of CSF can be diluted with autologous serum (final concentration, 11%)6 or with equal volumes of either 40% ethanol4 or 10% formalin5 to stabilize cells during transport to a referral laboratory for analysis. Dilution must be taken into account when cell concentrations are calculated.
Proteins in CSF are more stable than cells; thus, protein samples are stable overnight.4 Proteins should be measured biochemically in CSF (free of substances added to help preserve the cells) by using a microprotein assay. Proteins in CSF cannot be measured using a refractometer or other methods routinely used to measure serum protein concentration.4,5 Urine dipsticks can be used as a screening technique to estimate protein concentrations. Readings of 2+ or greater consistently detect CSF with significantly elevated protein concentrations; however, false-negative and false-positive results are common with trace or 1+ readings.6
Neutrophilic pleocytosis in CSF occurs secondary to trauma, infection (e.g., bacteria, fungi, rickettsia, protozoa), hemorrhage, neoplasia (e.g., meningioma), or SRMA.4,5 In dogs, marked neutrophilic pleocytosis is most commonly associated with the acute form of SRMA.1 Although uncommon, bacterial meningoencephalitis is an important differential diagnosis. In SRMA, neutrophils are nondegenerate and may be hypersegmented.4,5 No organisms are identified, and culture of CSF is negative.1 Bacterial meningoencephalitis can be diagnosed when neutrophils are degenerate, intracellular organisms are identified, or culture is positive.1,2,4,5 Unfortunately, organisms are not found in the CSF of most dogs with bacterial meningitis7 and a search for extraneural sites of infection is warranted.1 Diagnosis of SRMA is often based on clinical findings, laboratory findings, and response to immunosuppressive therapy. The dog in this report dramatically improved after glucocorticoid therapy but was lost to long-term follow-up.
Did You Answer ...
CSF should be colorless and clear. Erythrocytes should be absent, but small numbers frequently occur secondary to sample collection. Nucleatedcell concentration should beless than 5/µl and consist predominantly of lymphocytes and monocytoid cells. Neutrophils and eosinophils should comprise less than 10% and 1% of the nucleated cell population, respectively. Choroid plexus cells, ependymal cells, meningeal cells, andmacrophages may occasionally be present. Protein concentrationin CSF from cerebellomedullary and lumbar cistern collections should be less than 30 mg/dl and 45 mg/dl, respectively.4,5
The cytospin concentrated smear from CSF contained large numbers of nondegenerate neutrophils, many of which appeared hypersegmented. There were low numbers of small lymphocytes, monocytoid cells, and macrophages.
Marked neutrophilic inflammation has been associated with infectious meningoencephalitis, SRMA, and neoplasia.