Top 5 Minimally Invasive Surgical Procedures
Robert J. Hardie, DVM, DACVS, DECVS, University of Wisconsin–Madison
Rebecca Donnelly, DVM, University of Wisconsin–Madison
Some of the earliest reports of minimally invasive surgery (MIS) in veterinary medicine appeared in the literature 20 to 30 years ago and included procedures such as laparoscopic sterilization, laparoscopic liver biopsy, laparoscopic stapled gastropexy, and thoracoscopic partial pericardiectomy.1-7
Continued advances in medical technology, development of new surgical techniques in human medicine, growth of veterinary specialty training, and increased pet owner interest have continued to drive the growth of MIS in veterinary medicine.
MIS procedures are performed by introducing an optical scope (eg, laparoscope, thoracoscope, arthroscope) and specialized instruments into body cavities through small incisions (ie, ports) and placing them in locations specific to the surgical procedure. A camera attached to the optical scope captures video images that are displayed on a monitor and viewed by the surgical team during the procedure. For most laparoscopic procedures, the abdomen is insufflated with CO2 to increase the working space and allow maneuvering of surgical instruments during the procedure. (See Table for a detailed comparison of port positioning, CO2 insufflation, and materials for each procedure discussed.)
SUMMARY of THE TOP 5 Minimally Invasive Surgeries
*The rigidity of the thoracic wall combined with the limited pneumothorax that occurs with placement of the thoracic ports generally results in sufficient working space to resect the pericardium. If necessary, careful use of intermittent, low-pressure (2-3 mm Hg) insufflation may help reduce interference from the lungs during critical phases of the procedure, as long as the patient is monitored closely for complications from excessive atelectasis.
The benefits of MIS over conventional open surgical procedures are well documented and include decreased postoperative pain, more rapid return to function, and shorter hospitalization times.8-13 Surgeons benefit from improved magnification of the surgical field and better access to deep areas of the body. Disadvantages of MIS include the need for specific instrument training and the extended learning curve for some procedures. In addition, the instrumentation requires a substantial initial investment and the development of new sterilization and maintenance protocols.
Following are the authors’ most commonly performed MIS procedures. Several may have significant practical value for veterinary generalists with advanced training.
1. Laparoscopic Ovariectomy8-11
Laparoscopic ovariectomy allows for earlier return to function and decreased postoperative pain as compared with the conventional open surgical approach for ovariectomy.
After placement of the 2 (or 3) ports and insufflation of the abdomen, the patient (positioned in dorsal recumbency) is rolled or tilted to one side, and the contralateral uterine horn is identified. The cranial aspect of the uterine horn is grasped and retracted ventrally to expose the ovary. A transabdominal suture is placed through the proper ovarian ligament, suspending the ovary from the lateral abdominal wall (Figure 1). The ovarian vessels are sealed, and the suspensory and proper ovarian ligaments are transected to isolate the ovary. The patient is rotated to the opposite side, and the procedure is repeated for the other ovary. Each ovary is retracted through the instrument port while the transabdominal suture is released, then checked to ensure complete removal. The ovarian pedicles are inspected for adequate hemostasis, and the port incisions are closed routinely.
Potential complications include hemorrhage from incomplete ligation or sealing of ovarian pedicle vessels and iatrogenic injury to abdominal organs (eg, bladder, spleen, intestines) during port placement or instrument exchange. Laparoscopic ovariectomy is contraindicated in patients with uterine pathology (eg, pyometra, neoplasia) that could increase complications if surgery is performed laparoscopically and/or for which concurrent hysterectomy is warranted.
2. Laparoscopic-Assisted Cryptorchidectomy14,15
Laparoscopic-assisted cryptorchidectomy allows for better visualization of the entire caudal abdomen and enables identification of the precise location of the intra-abdominal testicle(s) with less surgical trauma as compared with the conventional open surgical approach via parapreputial incision.
After placement of a camera and abdominal insufflation, with the patient in dorsal recumbency, the caudal abdomen is explored, and the location of the testicle is confirmed (Figure 2A). A second port is placed to allow easy access to the testicle. The testicle is grasped (Figure 2B) and exteriorized through the instrument port. The vascular pedicle and ductus deferens are double ligated extracorporeally using routine techniques, and the ligated pedicle is returned to the abdomen. If necessary, the abdomen is re-insufflated and the procedure is repeated for the contralateral testicle. The pedicle is checked for adequate hemostasis, and the port incisions are closed routinely.


Cryptorchid testicle adjacent to the small intestine in the caudal abdomen (A) and elevation of the testicle using 5-mm grasping forceps before retraction through the port and extracorporeal ligation of the vascular pedicle (B)
Potential complications include entrapment of the testicle in the inguinal canal (requiring additional dissection to enable exteriorization), hemorrhage from incomplete ligation of testicular vessels, and iatrogenic injury to abdominal organs (eg, bladder, spleen, intestines) during port placement or instrument exchange. This procedure is contraindicated in patients with increased risk for possible seeding of a tumor if cryptorchid testicles are markedly enlarged or highly vascular because of neoplastic invasion.
3. Laparoscopic-Assisted Gastropexy16-18
Laparoscopic-assisted gastropexy requires smaller incisions and results in less surgical trauma as compared with the conventional open surgical approach.
After placement of the 2 ports and insufflation of the abdomen, with the patient in dorsal recumbency, the pyloric antrum is grasped midway between the greater and lesser curvature (Figure 3A), and the antrum is elevated to the right lateral abdominal wall. The length of the paracostal port incision is enlarged. The pyloric antrum is exteriorized, and 2 stay sutures are placed at the oral and aboral extents of the proposed gastropexy site to stabilize the stomach and maintain exposure. An incision is then made in the pyloric antrum, similar in location to that made for a conventional incisional gastropexy. Each edge of the seromuscular incision of the stomach is sutured to the transverse abdominus muscle in a simple continuous suture pattern using a slowly absorbing suture material. The internal and external abdominal oblique muscles are reapposed over the gastropexy site using a simple continuous pattern, followed by routine closure of subcutaneous tissue and skin. Once the abdominal wall is closed, insufflation is re-established and the gastropexy site is examined for proper positioning before routine closure of the camera port site (Figure 3B).


10-mm Babcock forceps grasping the pyloric antrum during a laparoscopic-assisted gastropexy (A) and completed gastropexy with the pyloric antrum (black arrow) sutured to the right lateral abdominal wall (B; white arrow)
Potential complications include kinking or obstruction of the pylorus due to improper positioning of the gastropexy site, body wall or intra-abdominal abscess formation caused by bacterial contamination from accidental penetration of the gastric lumen with the gastropexy sutures, and iatrogenic injury to abdominal organs (eg, bladder, spleen, intestines) during port placement or instrument exchange. This procedure is contraindicated in patients with severe peritoneal adhesions that prevent mobilization of the stomach to the right lateral abdominal wall.
4. Laparoscopic Liver Biopsy19, 20
Laparoscopic liver biopsy results in less surgical trauma as compared with the conventional open surgical approach. The complication rate is low, and samples obtained using laparoscopic cup forceps are considered satisfactory for histopathologic diagnosis.
After placement of 2 ports and insufflation of the abdomen, the liver is examined and the lesion or lobe(s) to be sampled are identified. The lesion is grasped, then held in place for 15 seconds before the biopsy sample is removed (Figure 4). The biopsy site should be monitored for excessive bleeding. After adequate hemostasis is ensured, the port incisions are closed routinely. The lateral recumbency position prevents complete visualization of the dependent lobes; however, it does not preclude performing biopsies, if necessary.

Laparoscopic liver biopsy using a 5-mm cup biopsy instrument. A minor degree of hemorrhage from the previous biopsy site can be seen.
Potential complications include uncontrollable hemorrhage from a biopsy site that requires conversion to an open approach for hemostasis, as well as iatrogenic injury to abdominal organs (eg, bladder, spleen, intestines) during port placement or instrument exchange. This procedure is contraindicated in patients with excessive ascites that cannot be drained adequately, as this results in poor visualization of the liver.
5. Thoracoscopic Partial Pericardiectomy13,21- 23
Thoracoscopic partial pericardiectomy allows for partial pericardiectomy with decreased postoperative pain, faster return to normal activity, and fewer incisional complications as compared with conventional open thoracotomy.
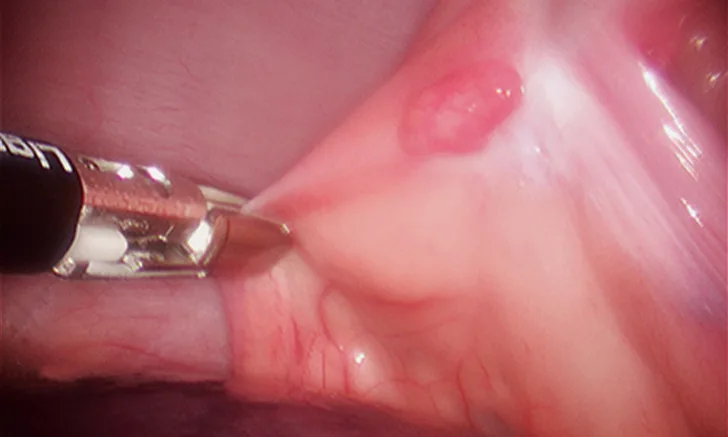
Following placement of 3 ports, with the patient in dorsal recumbency, the ventral mediastinum is incised. The ventral surface of the pericardium is identified, and any excess adipose tissue is removed. The ventral aspect of the pericardium is grasped and entered (Figure 5), and the ventral portion of the pericardium is removed (5- to 6-cm diameter window). The resected pericardium is then removed through one of the instrument ports. A thoracostomy tube is placed under direct visual guidance. The cut edge of the pericardium shoud be inspected for adequate hemostasis, and the port incisions are closed routinely.

5-mm grasping forceps retracting the cut edge of the pericardium during thoracoscopic pericardiectomy
Potential complications include hemorrhage, pneumothorax, or nerve damage caused by iatrogenic injury to the thoracic organs or phrenic or intercostal nerves during port placement or instrument exchange; respiratory or cardiovascular decompensation caused by pneumothorax or excessive insufflation; and cardiac arrhythmias caused by irritation of the heart during removal of the pericardium.
This procedure is contraindicated in small patients (<11 lb [5kg]) and patients with substantial adhesions of the lungs to the pericardium that limit working space or exposure to the ventral aspect of the pericardium. In addition, this procedure should not be performed in practices without a dedicated anesthetic support team to monitor and ventilate the patient during thoracoscopy.
Conclusion
These procedures are less traumatic alternatives to conventional open surgical approaches and can be easily added to a practice’s surgical repertoire, as long as the necessary equipment and support for anesthetic monitoring are available. Minimally invasive approaches may become the standard of care for certain procedures, and veterinarians will need to become proficient in these techniques to meet the evolving expectations of pet owners and other veterinary professionals.
MIS = minimally invasive surgery