Reduced Fertility & Epididymitis in a Dog
Cheryl Lopate, DVM, MS, DACT, Reproductive Revolutions, Aurora, Oregon
A 4.5-year-old intact male Parson Russell terrier was presented for an infertility evaluation. He had been bred to multiple bitches over the last 2.5 years.
History
In the first year of his breeding career, he had excellent fertility, with all bitches being bred naturally and conceiving with normal-sized litters of 4 to 8 puppies each. At the start of the second year of his breeding career, his fertility was normal. However, in the previous 6 months, he had been bred to 4 bitches (2 from his own kennel, 2 from outside kennels), none of which whelped any puppies. One aborted her litter on day 52 of pregnancy.
Examination
Physical examination revealed a temperature of 100.4° F, pulse at 120 bpm, respiration at 16 breaths/min, pink mucous membranes, and capillary refill time <2 seconds. There was mild retropharyngeal and submandibular lymphadenopathy. Auscultation of the heart and lungs was normal. Abdominal palpation was unremarkable. There was prominent scrotal dermatitis, and mild-to-moderate unilateral epididymal enlargement of the right epididymal head, body, and tail with concurrent thickening of the distal spermatic cord (Figure 1). Both testes were slightly softer than expected but of normal size.

Chronic epididymitis with the head, body, and tail of the epididymis as well as the distal spermatic cord (enlarged and irregular) postcastration; the testicle is smaller and flatter than normal because of chronic infection.
Diagnostic Results
Results of the semen evaluation, collected via manual stimulation, are provided below. There was no apparent pain associated with ejaculation.
Semen Evaluation
Further Diagnostic Testing
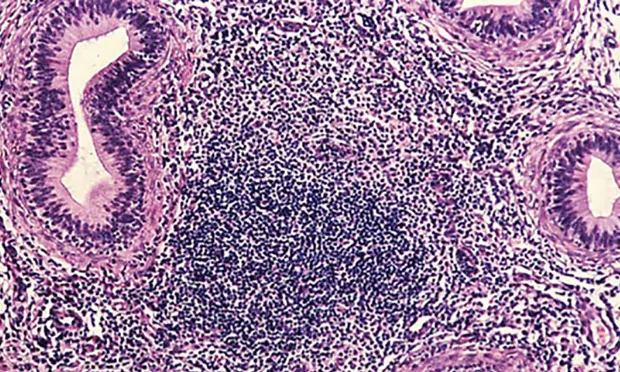
Enzyme-linked immunosorbent assay (ELISA) revealed a positive brucellosis status (Brucella canis infection), which was confirmed with agar gel immunodiffusion (AGID) testing. Qualitative semen culture was performed, and numerous B canis organisms were isolated along with moderate numbers of beta-hemolytic streptococci. The presence of white blood cells in semen cytology suggested a diagnosis of epididymitis. Histopathology demonstrates diffuse lymphocytic inflammation (Figure 2).

Prominent, diffuse lymphocytic inflammation commonly associated with B canis epididymitis. Images courtesy of Cornell Pathology Laboratory
Diagnosis
Canine brucellosis
Treatment & Outcome
The state veterinarian was notified because brucellosis is a reportable disease in most states. The dog was castrated and quarantined for 8 months and was treated with tetracycline and streptomycin for 8 weeks. Serologic testing was performed on the remaining animals in the kennel. Seventy-five percent of the animals in the original kennel (21/28) were positive for B canis. In the kennels in which outside bitches had been bred, 100% of the animals in 1 kennel and 50% in another (3 and 4 animals, respectively) were infected. All positive animals were neutered and treated with tetracycline (22-50 mg/kg) or were euthanized if the owner did not want to quarantine and treat.
Prevention
Serologic testing should be performed on all new arrivals to a breeding kennel, followed by 8 weeks of quarantine and retesting. Animals that are negative at this stage are considered safe to enter the general population and for breeding. Because brucellosis is a zoonotic disease, the importance of testing all new arrivals and other animals that are on the premises or that may have been exposed directly or indirectly cannot be overstated.
AGID = agar gel immunodiffusion, ELISA = enzyme-linked immunosorbent assay, PCR = polymerase chain reaction, TAT = tube agglutination test, WBC = white blood cell