Fenbendazole is a broad-spectrum benzimidazole dewormer that is safe and effective for dogs when used according to labeled directions.
Clinical Applications
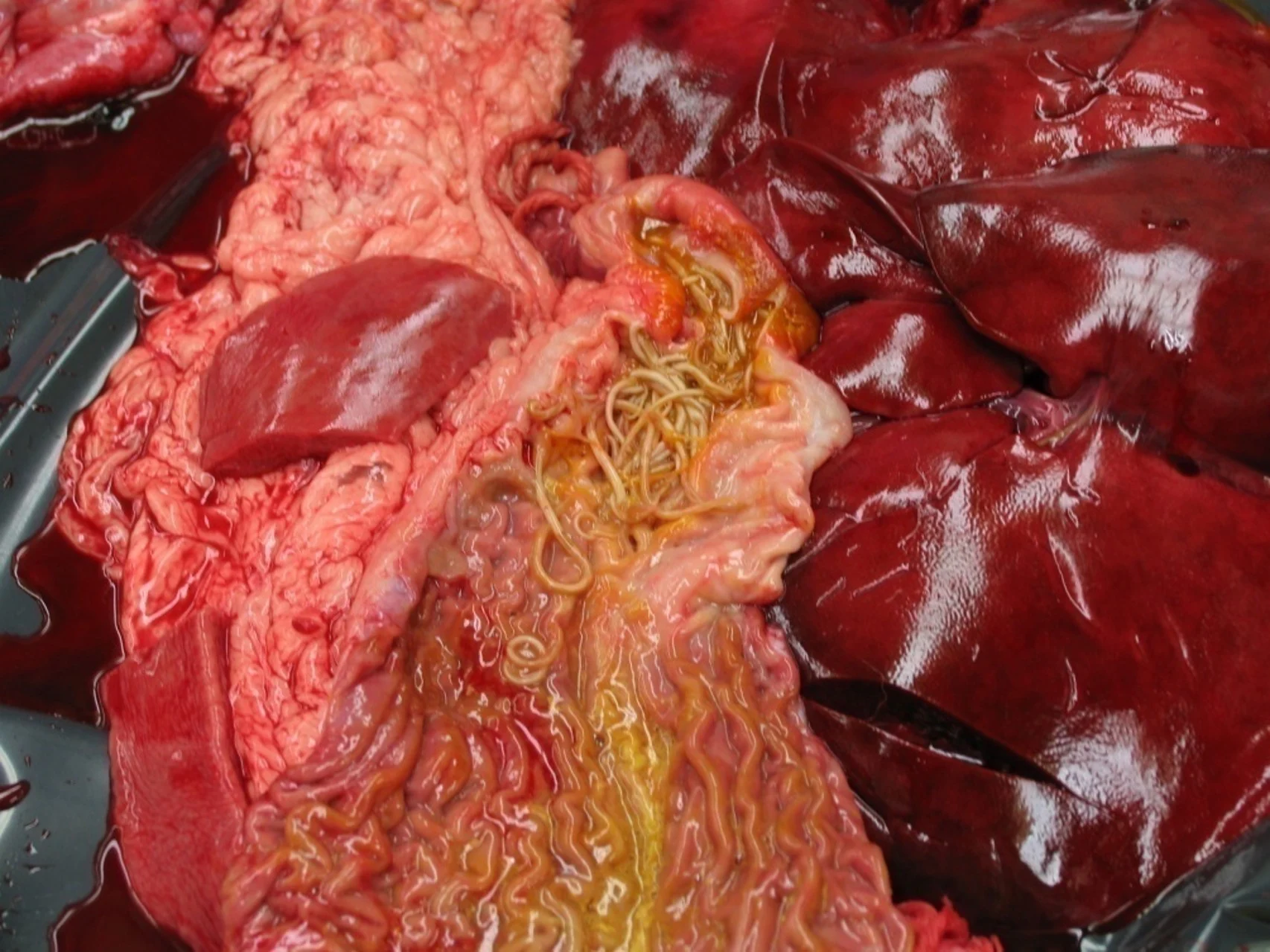
Fenbendazole is FDA-approved for the treatment and control of roundworms (Toxocara canis, Toxascaris leonina), hookworms (Ancylostoma caninum, Uncinaria stenocephala), whipworms (Trichuris vulpis), and tapeworms (Taenia pisiformis) in dogs.
Commercially, fenbendazole is positioned as an intestinal parasite control product and a complement to monthly heartworm preventives and flea and tick control products, as some of these agents do not protect against all 4 major types of intestinal parasites.
Dosage Information
Fenbendazole granules (222 mg/g [22.2%]) are available in 1-lb (454-g) jars and are dispensed at 1 g per 10 lb (4.44 kg) of body weight. Granules are also available in 1-, 2-, and 4-g packets for treating 10-, 20-, and 40-lb of body weight, respectively.
The labeled dosage (50 mg/kg [22.7 mg/lb] PO once daily in food for 3 consecutive days) may be less convenient for pet owners compared with single-dose regimens. Granules should be mixed in a small amount of the patient’s usual food. Dry kibble can be moistened to facilitate mixing with granules.
Extra-Label Uses
Fenbendazole (50 mg/kg PO once daily for 3-5 days) is used extra label for treatment of Giardia spp in dogs and cats, but there is no evidence to support treatment beyond 3 days, and extended treatments are associated with toxicity (see Cautions).1 A study demonstrated fenbendazole to be as effective as metronidazole when both drugs were administered to dogs at a dosage of 50 mg/kg PO every 24 hours for 5 days2; however, metronidazole administered at >40 mg/kg has been associated with neurotoxicity.3
For extra-label treatment of lungworms in dogs (Oslerus osleri, Eucoleus aerophilus [formerly Capillaria aerophila], Crenosoma vulpis) and cats (Aelurostrongylus abstrusus), the recommended dosage is 50 mg/kg PO once daily for up to 14 days.4 For extra-label treatment of trematodes in dogs (Nanophyetus salmincola, Paragonimus kellicotti, Paragonimus westermani, Heterobilharzia americana) and cats (N salmincola), the recommended dosage is 50 mg/kg PO once daily for 10 to 14 days.4-6
Resistance
Monitoring anthelmintic efficacy is uncommon in companion animal practice7; therefore, neither the potential for emerging resistance to intestinal parasites nor the necessity of anthelmintic stewardship has received sufficient attention.8,9
Reports of fenbendazole resistance in A caninum and T vulpis are increasing in frequency.8-10 Multiple-drug resistance in A caninum is common, and dogs may have persistent hookworm infections despite multiple anthelminitc treatments.
Cautions
Fenbendazole is generally considered safe and effective when administered to dogs according to the labeled directions. Fenbendazole can also be safely used in many other species for the treatment and control of various nematode infections.
Of the benzimidazole dewormers, albendazole has been noted most often to cause bone marrow hypoplasia in humans, dogs, and cats.11-13
Some case reports have documented bone marrow hypoplasia or pancytopenia in dogs treated with fenbendazole at extra-label doses (50 mg/kg PO every 12 hours) and/or with increased treatment durations (5-14 days).14-16 The Center for Veterinary Medicine began monitoring cases of bone marrow hypoplasia and pancytopenia in dogs treated with fenbendazole for extra-label treatment durations.17 As of October 2023, the organization had received 12 reports in dogs treated for 5 to 14 days. Fenbendazole-associated bone marrow hypoplasia has been reported in birds (eg, vultures, storks, pigeons, doves) and in North American porcupines.18-21
Although the exact mechanism behind benzimidazole-associated bone marrow hypoplasia has not been determined, bone marrow cells are susceptible to drug toxicity, as the cells divide rapidly and are metabolically active. Benzimidazoles bind to tubulin, a structural protein of microtubules, and may have adverse effects on rapidly dividing cells. Discontinuation of fenbendazole typically leads to resolution of clinical signs and hematologic abnormalities.14-16
There are no published reports of fenbendazole-associated bone marrow hypoplasia or pancytopenia in cats.