Canine & Feline Coagulopathy
PROFILE
Definition
Coagulopathy is a condition in which the blood’s ability to clot is impaired, leading to hemorrhage or thrombosis.
The following focuses on hemorrhagic presentations.
Any of these areas can be affected:
Failure of primary hemostasis, or formation of the initial platelet plug1
Decreased platelet numbers or function or reduced von Willebrand factor (vWF) can lead to mucosal bleeding and bruising.
Failure of secondary hemostasis, or formation of a stable fibrin clot via a cascade of enzymes that ultimately convert fibrinogen to fibrin1
Defects can lead to severe bleeding diatheses.
Excessive fibrinolysis, or plasmin breakdown of a fibrin clot1
Excessive clot breakdown can result in prolonged bleeding or delayed rebleeding.
Related Article: Evaluating the Bleeding Patient: Point-of-Care Tests
Systems
Bone marrow: Production of platelets by megakaryocytes
Liver: Production of all clotting factors and carboxylation of factors II, VII, IX, and X via a vitamin K–dependent enzyme
GI tract: Absorption of vitamin K in the presence of fat and bile
Endothelium: Production of vWF
Site of hemorrhage: Exposure of tissue factor initiates platelet activation and coagulation cascade.
Platelets: Provide the membrane surface for coagulation cascade and secrete granules that contain ingredients for clotting (ie, vWF, factor VIII)
Genetic Implications
Numerous inherited bleeding disorders exist in dogs and cats2-5 (see handout Inherited Coagulopathy: Commonly Affected Breeds).
vWD is most common in dogs.1,2,5
It is often seen with unexplained bleeding in young dogs with or without trauma.
There may also be suspicion with excessive bleeding after planned trauma (eg, neutering).
Geographic Distribution
Infectious causes may vary by region (see table Causes of Thrombocytopenia).
Related Article: Current Thoughts on Coagulopathy Testing
Signalment
Breed Predilection
Seen with inherited coagulopathy
Cocker spaniels, poodles, and Old English sheepdogs are overrepresented in patients with immune-mediated thrombocytopenia.5
Age & Range
Any age, but differentials may change based on age (see chart Coagulopathy in Juvenile Patients)
Related Article: Just Another Rat Bait?
Causes
Disorders of Primary Hemostasis
Thrombocytopenia (see table Causes of Thrombocytopenia)1,5
Decreased production, increased destruction or consumption, and sequestration
Platelet count must fall below ~50,000/µL for spontaneous bleeding to occur.
Thrombocytopathia
Inherited
Acquired
Caused by certain drugs5 (see handout Inherited Coagulopathy: Commonly Affected Breeds), uremia, hepatic failure, and myeloproliferative disorders
vWD is caused by inherited structural and quantitative deficiencies of vWF.2
Related Article: Practical Canine Blood Transfusion
Disorders of Secondary Hemostasis
Anticoagulant rodenticide toxicity
Brodifacoum is most common.6
Inhibits vitamin K1 epoxide reductase resulting in dysfunction of factors II, VII, IX, and X and proteins C and S6-8
Hepatic disease
Decreased or abnormal coagulation factor synthesis
Cholestatic disease
Decreased absorption of vitamin K can cause dysfunctional forms of factors II, VII, IX, and X.
Inherited factor deficiencies
Factor VIII deficiency (ie, hemophilia) is most common.2,4
Disorders of Fibrinolysis
Postoperative bleeding in greyhounds9
Disorders Affecting All Aspects of Coagulation
Disseminated intravascular coagulation (DIC)
Early stage is characterized by thrombosis; late stages result in hemorrhage.
Bleeding after excessive consumption of endogenous platelets/clotting factors1
DIC is secondary to an underlying issue (eg, severe trauma, neoplasia, sepsis, overwhelming inflammation).
History & Examination
A thorough history is essential and can guide diagnostics10:
Signalment
Duration and progression of signs
Recent trauma or surgery
Bleeding events (eg, teething, vaccination, elective surgery)
Evidence of bleeding at multiple sites
Previous transfusions
Medication history
Toxin exposure
Travel history

Clinical Signs
Clinical signs related to sites of bleeding or systemic signs of blood loss
Primary hemostasis
Capillary or small vessel hemorrhage1,5,10
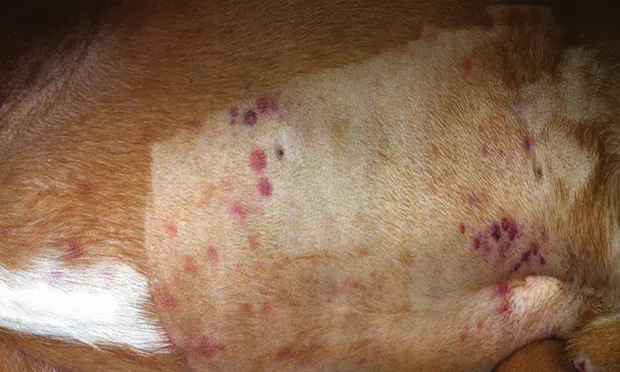
Petechiae (Figure 1) or ecchymosis
Mucosal hemorrhage (ie, epistaxis, hematuria, gingival bleeding, hematemesis, melena, and hemoptysis)
Secondary hemostasis
SC or cavitary bleeding1,5,10
Single or multiple hematomas
Dyspnea
Dull lung or heart sounds
Abdominal distention
Lameness
Fibrinolysis
Excessive bruising 12–24 hours after surgery or injury
General blood loss
Lethargy, inappetence, collapse, pale mucous membranes, tachycardia, and bounding or weak pulses
Figure 1. Petechiation in a boxer (4 years of age) with immune-mediated thrombocytopenia. Note bruising in the unclipped region.
DIAGNOSIS
Definitive
Results of laboratory coagulation testing are essential for disease classification (see table Coagulation Testing for Hemostatic Defect Type).
Differentials
Unwitnessed trauma, neoplasia, postsurgical complications (eg, ligature slippage)
Laboratory Findings & Imaging
CBC and blood smear
Platelet estimate, presence of anemia/leukopenia, intracellular organisms, and RBC morphology
Chemistry panel
Evaluation of total protein concentration and liver function studies
Buccal mucosal bleeding time (BMBT)1,10,11
Prolongation
Abnormal platelet function or vasculitis
Should be performed with a normal platelet count as thrombo-cytopenia alone may result in a prolonged or abnormal test result.
vWF activity5,10
To diagnose and characterize type of vWD
Prothrombin time (PT)1,10
Evaluates extrinsic (factor VII and tissue factor) and common pathways
Can be measured 48–72 hours after known exposure to anticoagulant rodenticides (without vitamin K administration)
If results are within reference ranges, vitamin K therapy is not required.
Activated partial thromboplastin time (aPTT)1,10
Evaluates intrinsic (ie, factors VIII, IX, XI, XII) and common (ie, factors I [fibrinogen], II, V, X) pathways
Prolonged aPTT with normal PT suggests specific intrinsic factor abnormalities.
Activated clotting time (ACT)1,10
Evaluates intrinsic and common pathways
Less sensitive than aPTT
High-performance liquid chromatography (HPLC)6
Recommended if exposure to anticoagulant rodenticides is suspected but not confirmed, especially when other differentials are likely (eg, hemangiosarcoma)
Individual factor analysis10
Performed to identify inherited factor deficiencies
Tests of fibrinolysis10
Include fibrinogen assays, thrombin time, fibrin degradation products, and d-dimers
Infectious disease screening
Cases with primary hemostatic defects, fever, and/or generalized illness should be screened for Ehrlichia canis, Anaplasma phagocytophilum, A platys, and Rickettsia rickettsii by measuring antibody titers or conducting a PCR assay as indicated by geographic location.
Bone marrow aspiration/biopsy5
Indicated with thrombocytopenia if other cell lines (RBC or WBC) are unusually increased or decreased

Radiography and abdominal ultrasonography
Survey radiography is indicated for pulmonary, pleural, or abdominal hemorrhage (Figures 2A and B).
For primary disease processes in patients with DIC (eg, masses, pneumonia)
Advanced imaging
CT scan or MRI in cases of CNS hemorrhage

Figure 2A. Lateral thoracic radiograph of a Welsh corgi (9 years of age) following ingestion of an anticoagulant rodenticide at least 3 days before presentation. Note the mixed pattern of alveolar infiltrates and scant pleural effusion (hemorrhage).
Figure 2B. VD radiograph of the patient. Alveolar pattern in the right cranial and middle lung lobes represents one of the radiographic manifestations of pulmonary hemorrhage.
TREATMENT
Inpatient
Shock
Intravascular volume support
Transfusion as indicated
Outpatient
Within 24 hours following ingestion of anticoagulant rodenticides and if patient is systemically normal
Can be performed in a thrombocytopenic patient if it is eating and drinking well and not severely anemic
Medical (see Medications)Primary Goals
Stabilizing shock patients with IV fluids
Packed RBCs or fresh whole blood may be necessary.
Secondary Goals
Arresting bleeding, if possible
Transfusing missing clotting factors (fresh-frozen plasma or cryoprecipitate)
Encouraging platelet formation if thrombocytopenic (eg, administration of vincristine)
Managing local bleeding if possible (eg, wrap distensible areas, excising bleeding masses as soon as is safe)
Tertiary Goals
Treating underlying cause
Treatments will be diagnosis-specific and may include immunosuppressive medication, vitamin K1, or antibiotics.
Client Education
Clients should be advised to restrict activity if patient is at risk for spontaneous bleeding or is weak from previous bleeding.
Inherited coagulopathy
Clients should be informed that the patient may require numerous transfusions throughout its life and should not be bred.
Immune-mediated thrombocytopenia
Clients should be educated about the risk for relapse and to avoid future antigenic stimulation (eg, vaccines).
M**EDICATIONS**

Drugs & Fluids
IV isotonic crystalloids
Boluses should be administered in increments of 20–30 mL/kg of lactated Ringer’s solution or 0.9% saline solution until heart rate, blood pressure, mucous membrane color, and mental status are normal.
After resuscitation, IV fluids should be continued at rates accounting for ongoing loss, dehydration, and maintenance needs (often 3–6 mL/kg/h but will vary).
Bolus infusion of acetate-containing fluids (eg, P-lyte) is not recommended; rapid infusions of acetate can cause vasodilation and hypotension.12,13
Hypertonic saline (7.2%–7.5% NaCl)
Effective only if the patient is not already dehydrated or hypernatremic
2–4 mL/kg administered no faster than over 15 minutes, followed by isotonic crystalloids
Blood transfusion
Indicated if the patient still shows signs (eg, tachycardia, abnormal pulses) or weakness after fluid resuscitation
PCV of <20% after an episode of acute bleeding warrants transfusion consideration.
Whole blood: 10–22 mL/kg IV14
Packed RBCs: 6–10 mL/kg IV14
Fresh-frozen plasma
Supplies clotting factors rapidly (XII, XI, X, IX, VIII, VII, V, II, vWF, fibrinogen)
Indicated for hemophilia, vWD, anticoagulant rodenticide ingestion with hemorrhage
6–10 mL/kg IV14
Cryoprecipitate
Indicated for vWD
1 U/10 kg14
Desmopressin acetate
Stimulates release of vWF
Indicated in patients with vWD
1 µg/kg SC 1 hour before surgery5
Vitamin K
Oral formulation is ideal for anti-coagulant rodenticide.
May enhance absorption when given with a fatty meal
Injectable form is poorly absorbed but necessary for cases when malabsorption of vitamin K is suspected (ie, cholestasis, liver failure, shock).
Rodenticide
Vitamin K at 3–5 mg/kg PO (ideal) divided q12h for 4 weeks7,15
PT should be rechecked 48 hours after last dose.
Not required after acute ingestion and decontamination if PT is normal 48–72 hours after exposure16
Malabsorption
0.5–1 mg/kg SC q12–24h for 3 doses
Antifibrinolytic agents (eg, amino-caproic acid)
Used in greyhounds to prevent postsurgical bleeding9
Doxycycline
Used in cases of infectious thrombocytopenia
5–10 mg/kg PO q12h
Immunosuppressive medications (eg, prednisone, azathioprine, cyclosporine, leflunomide)17
Used to treat immune-mediated thrombocytopenia
Decontamination
Apomorphine administered at 0.03 mg/kg IV and activated charcoal at 1–4 g/kg PO for recent (ie, within 1–3 hour) rodenticide ingestion18
Precautions & Interactions
Blood typing and crossmatching is indicated in patients receiving multiple transfusions.
FOLLOW-UP
Patient Monitoring
For recurrence of clinical signs or further bleeding episodes
Patient may need ongoing laboratory monitoring.
At-Home Treatment
Strict rest with padded bedding to prevent rebleeding until risk has passed (ie, platelet count, PT, and/or aPTT have returned to normal as indicated)
Clients should inform any veterinary providers about prior transfusions and history of coagulopathy.
IN GENERAL
Relative Cost
Recent anticoagulant exposure with immediate decontamination: $
Inherited coagulopathy, if diagnosed before severe bleeding event: $$$
Any coagulopathy with severe bleeding: $$$$$
Prognosis
Prognosis varies based on coagulopathy type and underlying cause.
Thrombocytopenia
Infectious: good
Immune mediated: fair
Hereditary coagulopathy
Primary hemostatic defects (eg, vWD): normal lifespan possible, although multiple transfusions may be required
Type III vWD and inherited factor: variable
Some live full lifespans while others have multiple bleeding events.
Anticoagulant rodenticides
Recent exposure and decontamination: excellent
3–5 days postexposure with bleeding: good, but treatment more costly
DIC
Guarded, unless the underlying cause can be rapidly corrected
Future Considerations
Point-of-care testing (eg, PFA-100, thromboelastography [TEG]) is not widely available but may become useful in future diagnosis and treatment.
_vWD = von Willebrand disease, vWF = von Willebrand factor, aPTT = activated partial thromboplastin time, BMBT = buccal mucosal bleeding time, DIC = disseminated intravascular coagulation, PT = prothrombin time_
CANINE & FELINE COAGULOPATHY • Michelle Fulks & Virginia Sinnott
References
1. Bleeding disorders. Hackner SG. In Silverstein DC, Hopper K (eds): Small Animal Critical Care Medicine—St. Louis: Saunders Elsevier, 2009, pp 507-514.2. Inherited disorders of hemostasis in dogs and cats. Barr JW, McMichael M. Top Companion Anim Med 27:53-58, 2012.3. Inherited platelet disorders. Boudreaux MK. JVECC 22:30-41, 2012.4. Inherited coagulopathies. Carr, AP. In Ettinger SJ, Feldman EC (eds): Veterinary Internal Medicine, 6th ed—St. Louis: Saunders Elsevier, 2005, pp 1929-1933.5. Platelet disorders. Brooks MB, Catalfamo JL. In Ettinger SJ, Feldman EC (eds): Veterinary Internal Medicine, 6th ed—St. Louis: Saunders Elsevier, 2004, pp 1918-1928.6. Rodenticides. Murphy MJ. Vet Clin North Am Small Anim Pract 32:469-484, 2002.7. Common rodenticide toxicoses in small animals. DeClementi C, Sobczak BR. Vet Clin North Am Small Anim Pract 42:349-360, 2012.8. Acquired coagulopathies. DuFort RM, Matros L. In Ettinger SJ, Feldman EC (eds): Veterinary Internal Medicine, 6th ed—St. Louis: Saunders Elsevier, 2004, pp 1933-1937.9. Retrospective evaluation of the effectiveness of epsilon aminocaproic acid for the prevention of postamputation bleeding in retired racing Greyhounds with appendicular bone tumors: 46 cases (2003-2008). Marin LM, Iazbik MC, Zaldivar-Lopez S, et al. JVECC 22:332-340, 2012.10. Diagnostic approach to small animal bleeding disorders. Herring J, McMichael M. Top Companion Anim Med 27:73-80, 2012.11. Assessment of platelet function. Jandrey KE. JVECC 22:81-98, 2012.12. Sodium acetate, an arterial vasodilator: Haemodynamic characterisation in normal dogs. Saragoça MA, Bessa AM, Mulinari RA, et al. Proc Eur Dial Transplant Assoc Eur Ren Assoc 21:221-224, 1985.13. Comparison of the hemodynamic effects of sodium acetate in euvolemic dogs and in dogs submitted to hemorrhagic shock. Saragoça MA, Mulinari RA, Bessa AM, et al. Braz J Med Biol Res 19:455-458, 1986.14. Blood transfusions and blood substitutes. Hohenhaus AE. In Dibartola SP (ed): Fluid, Electrolyte, and Acid-Base Disorders in Small Animal Practice—St. Louis: Saunders Elsevier, 2012, pp 585-604.15. Rodenticides. Brown AJ, Waddell LS. In Silverstein DC, Hopper K (eds): Small Animal Critical Care Medicine—St. Louis: Saunders Elsevier, 2009, pp 346-349.16. Incidence of prolonged prothrombin time in dogs following gastrointestinal decontamination for acute anticoagulant rodenticide ingestion. Pachtinger GE, Otto CM, Syring RS. JVECC 18:285-291, 2008.17. Therapeutic options for immune-mediated thrombocytopenia. Nakamura RK, Tompkins E, Bianco D. JVECC 22:59-72, 2012.18. Approach to poisoning and drug overdose. Schildt JC, Jutkowitz LA. In Silverstein DC, Hopper K (eds): Small Animal Critical Care Medicine—St. Louis: Saunders Elsevier, 2009, pp 326-329.