Canine Atopic Dermatitis: Supporting the Multimodal Approach
Sponsored by Nutramax Laboratories
Canine atopic dermatitis (AD) is a life-long condition that often manifests relatively early in life (ie, between 6 months and 3 years of age) and affects ≈1 in 10 dogs.1-3 Predisposed breeds include West Highland white terriers, Lhasa apsos, boxer dogs, German shepherd dogs, cocker spaniels, bulldogs, shar-peis, and golden and Labrador retrievers, some of the most popular dog breeds.2-4
Key Points
CAD is a life-long condition that affects ≈1 in 10 dogs1-3 and can be difficult to manage and frustrating for owners and veterinarians alike.
Traditional approaches to CAD may be only partially effective alone, are sometimes cost-prohibitive, and can potentially cause adverse effects.
Supplemental options, including hardy kiwi extract, ceramides, β glucan, and essential fatty acids, provide an opportunity to support the efforts of a multimodal approach, potentially reduce pharmaceutical use, and promote maintenance of a healthy skin barrier.
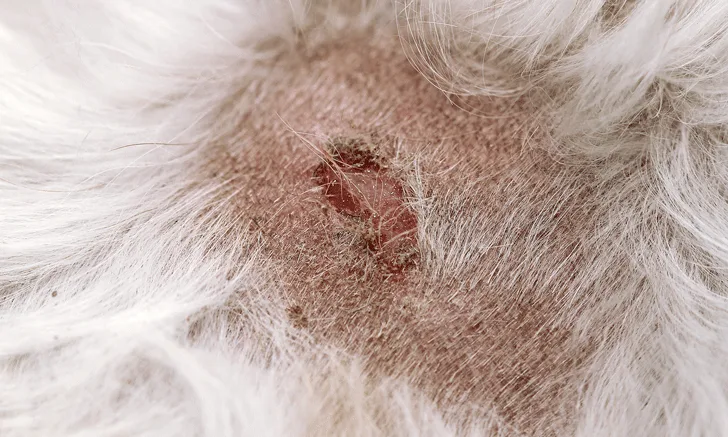
AD can impact patient and owner quality of life.5 Signs of AD include pruritus, erythema, and development of other skin lesions and secondary skin infections.1,2 The most common body regions affected include the axillae, inguinal area, interdigital spaces, muzzle, concave pinnae, antebrachial fossae, and periocular areas.6
Self-trauma and skin infections can exacerbate inflammation.3Secondary skin and ear infections from Staphylococcus spp and/or Malassezia spp are common and tend to recur, which exacerbates the damaging cycle in the skin that perpetuates the condition.7This propensity for relapse, as well as AD’s attribution to genetic and environmental factors, makes AD similar to its counterpart eczema in human medicine.8-11
Pathogenesis
AD is thought to be caused by an immunoglobulin E (IgE)-mediated hypersensitivity immune response; these allergy response-associated antibodies are found mostly in the skin, mucous membranes, and lungs. AD has traditionally been thought to be caused by a response to inhaled allergens (eg, pollen, dust, dander, mold)2; however, newer evidence in dogs suggests that the cutaneous route of exposure to allergens is the most important.10 The underlying cause of AD is not fully understood,1 but increasing evidence shows that skin barrier dysfunction is prevalent in atopic dogs.6 It is unknown whether the skin barrier defect is a primary or secondary issue.12 Evidence suggests that inflammation disrupts skin barrier properties and likely contributes to a vicious cycle in dogs with AD in which an abnormal skin barrier increases the propensity toward allergen sensitization and exposure and then skin barrier function is worsened further by allergic inflammation response.6,7,13 These issues can also lead to secondary bacterial and yeast skin infections, which further exacerbate the cycle.1,2 Common components of skin barrier dysfunction include skin dysbiosis, structural changes, and altered intercellular lipid content, all of which allow for transepidermal water loss (TEWL) and penetration by allergens.7,9 Studies have shown that TEWL is greater in atopic dogs than in normal dogs, increases with allergen exposure in atopic dogs, is greater in younger atopic dogs than in adult atopic dogs after exposure, and is lower during remission as compared with atopic dogs prior to treatment.10,14 Allergen penetration activates an immune response that exacerbates inflammation and clinical signs primarily through degranulation of histamine-releasing mast cells, although other inflammatory mediators and immune cells are also involved.3
The Role of Intercellular Lipids
Altered intercellular lipids contribute to increased TEWL and penetration by allergens.15 In addition to free fatty acids, cutaneous ceramides are a major lipid component of the stratum corneum (ie, outer skin layer).15,16 As compared with normal dogs, dogs with AD have decreased levels of both free fatty acids and cutaneous ceramides in skin lesions and skin that looks grossly normal.13 In a study, after being challenged with allergens, ceramide levels in atopic dogs returned to prechallenge levels within 2 months of lesion remission.17
Traditional Approaches
AD is usually managed through various therapy combinations. A number of products, including Janus kinase inhibitors, monoclonal antibodies, and corticosteroids or immunosuppressants, are traditionally used in the management of canine AD. Allergen-specific immunotherapy is the only treatment option that may lead to allergen tolerance over time18; although a safe option, allergenspecific immunotherapy can be costly and take 6 to 9 months for initial observations of efficacy.1
Supplemental Options
Other options for AD may be costprohibitive to the pet owner, cause adverse effects, and/or be incompletely effective alone.1,19 Topicals and antihistamines can be less expensive but tend to have lower efficacy and duration of effect.1,2,20 Therefore, research into additional supplemental management options for AD could be beneficial.
Hardy Kiwi
Extracts derived from hardy kiwi (Actinidia arguta) fruit have been shown to change Canine Atopic Dermatitis Extent and Severity Index (CADESI)-03 scores in atopic dogs when used with steroids. CADESI is a subjective scoring system used to assess the severity of AD and monitor the condition over time, especially in clinical trials.21 Hardy kiwi extract has also been used to maintain lower CADESI scores after steroids have been tapered off.1
This maintenance is likely achieved through the effects of the extract shown in regulation of allergic response and inflammatory mediators.1,11,22-28 The fruit contains phenolic acids (ie, quinic and citric acid), monoterpenes, and flavonoids that may contribute to the activity of its extracts.27 The duration of administration has been correlated positively with the effects and benefits. Hardy kiwi extract is best used long-term as part of a multimodal approach to therapy.1,22
Ceramides
Ceramides are commonly considered for topical application, but evidence for oral administration is emerging.12,29-32 Ceramides present in the skin represent 35% to 40% of the intercellular cement that binds the cells of the stratum corneum to provide an effective skin barrier.12 Positive effects with oral ceramides in humans have been demonstrated on skin hydration and TEWL, IgE reduction, and reduction of other inflammatory mediators.12,29-33
β Glucans
β glucans are found in nature as a structural component of the cell wall of yeast, bacteria, and fungi.34 They are known for their immune-modulating effects and may help activate both innate and adaptive responses involved in AD.34,35 In a double-blinded, placebo-controlled study in dogs with AD, mean overall improvement of pruritus, erythema, scaling, and lichenification was 63%.36 These effects are likely associated with the modulation of inflammatory mediators.37
Essential Fatty Acids
Because of their safety and low cost, essential fatty acids are a common component of a multimodal approach to managing canine AD.1,8 Inflammatory mediator, pruritus, and drug-sparing benefits have been demonstrated.38-40 Linoleic acid is commonly used, as it is the precursor to γ linolenic acid; however, due to the absence of appropriate enzymes, this conversion is not possible in the skin and overall may be systemically deficient in atopic patients.3,20,40-42 Providing performed long-chain omega-3 fatty acids and γ linolenic acid has had effects on TEWL, pruritus, IgE levels, inhibition of histamine release, and modulation of inflammatory mediators involved in AD.3,20,40-47
Conclusion
Canine AD is a chronic condition that can be difficult to manage and frustrating for owners and veterinarians alike. Traditional approaches may be only partially effective alone, are sometimes cost-prohibitive, and can potentially cause adverse effects. Supplemental options, including hardy kiwi extract, ceramides, β glucans, and essential fatty acids, such as Dermaquin, provide an opportunity to support the efforts of a multimodal approach, potentially reduce pharmaceutical use—and therefore possible reduction of adverse effects—and promote maintenance of a healthy skin barrier in a cost-effective manner.