Biofilms & Urinary Tract Infections…A Sticky Situation
You have asked…What is a biofilm, and how does it affect the urinary tract of animals?
The experts say…
A biofilm is a structured community of bacterial, fungal, or other cells enclosed in a self-produced polymeric matrix adherent to an inert or living surface.1 Biofilms are common in living organisms, including animals, but can also be found in other environments. Biofilms alter the function and pathogenicity of bacteria. Growing evidence suggests they may play a substantial role in infections, especially those that are recurrent or difficult to treat.2
From the perspective of bacteria, becoming part of a biofilm confers certain advantages.
From the perspective of bacteria, becoming part of a biofilm confers certain advantages. Within the biofilm, bacteria are able to pool their resources and receive protection from the immune system, antimicrobials, harsh environments and other stressors. However, being part of a community may also decrease access to water and oxygen, especially at depths further from the surface, and lead to an accumulation of waste products.3 Adjusting to these disadvantages may ultimately enable bacteria to thrive in this environment and increase problems for veterinary patients.
Related Article: Differentiating Canine Urinary Tract Infections
Bacteria change from free-living (or planktonic) organisms to ones that can adhere to surfaces (ie, to establish a colony) in order to form a biofilm; in doing so their behavior and structure alter. As the community grows, autoinducers, chemical-signaling molecules that enable bacteria to sense one another and regulate one another’s activities, accumulate. As they accumulate, the autoinducers induce changes in bacterial surface attachments, the extracellular polymeric matrix, and the amount and type of virulence factors that are expressed. Alterations in gene expression lead to phenotypic changes in flagella, the structure of cell walls, and the production of enzymes. Up to 40% of cell wall proteins appear to be different in bacteria found in a biofilm compared with their planktonic counterparts.4
Alterations in the normal structure of the lower urinary tract, however, can make it easier for bacteria to adhere, grow, and create a probiofilm environment. Any event that causes trauma to the mucosal layer of the bladder and erodes the glycosaminoglycan layer can result in a disruption in these natural defenses.
Alterations in the normal structure of the lower urinary tract, however, can make it easier for bacteria to adhere, grow, and create a probiofilm environment. Any event that causes trauma to the mucosal layer of the bladder and erodes the glycosaminoglycan layer can result in a disruption in these natural defenses.
Most of what we know about the interactions between antimicrobials and bacteria are based on planktonic bacteria. However, biofilm bacteria can be up to 1000 times more resistant to antimicrobials than bacteria that are free-living.5 Antimicrobials may not penetrate deeply into the extracellular matrix or may be thwarted by the anaerobic interior. Increased antibiotic resistance develops because the proximity of bacteria increases their ability to share resistance genes with each other. In addition, at any time 1% to 10% of cells within a biofilm slow down because of altered metabolism and expression.5 Dormant cells exhibit a slower metabolism and are more resistant to antimicrobials. Dormancy is reversible, and when environmental changes occur, these cells can revert to a more active form.5
Related Article: Recurrent Urinary Tract Infection
How Biofilms Affect the Urinary Tract

In a healthy animal, the lower urinary tract has many natural defenses that prevent the development of infections. Periodic emptying of the bladder, sloughing of epithelial cells that line the urinary system, and the mucous layer lining the epithelium prevent bacteria from staying in the urinary system. The body’s natural immune response, low availability of iron (which is necessary for bacteria to function), and high concentration of urea also make the urinary system inhospitable to bacteria.6
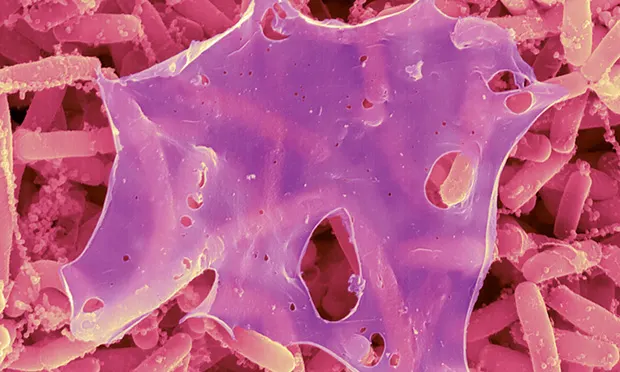
Image 1. Staphylococcus aureus biofilm on an indwelling catheter
Alterations in the normal structure of the lower urinary tract, however, can make it easier for bacteria to adhere, grow, and create a probiofilm environment. Any event that causes trauma to the mucosal layer of the bladder and erodes the glycosaminoglycan layer can result in a disruption in these natural defenses. Such events include placement of a urinary catheter, the presence of stones, or masses within the bladder or urethra. In animal models of urinary tract infections (UTIs), bacteria instilled directly into the bladder of healthy animals were cleared in 2 to 3 days by natural mechanisms. However, the presence of a surgically placed foreign object in the bladder caused animals to develop chronic urinary tract infections with a concurrent biofilm on the foreign object in less than 24 hours.7
If a biofilm infection is suspected, treatment strategies include prompt removal of implants or foreign material in combination with appropriate antimicrobial therapy.
Recognizing Biofilms
Biofilms tend to develop in the urinary tract—but how do we detect the presence of a biofilm?
Unfortunately, biofilms are difficult to detect. Visualization techniques used in research include scanning electron microscopy or confocal laser microscopy, neither of which is readily available or useful to detect infection in a live animal. A polymerase chain reaction (PCR) test to search for a biofilm-specific gene is in development but is not yet commercially available.8
Suspect the presence of a biofilm under the following conditions:
Any chronic urinary tract infection, especially when the patient presents with a low bacterial cell count.
Relapses occur after theoretically successful treatment.
Antibiotic use fails to clear signs in culture-directed treatment.
Any catheter-associated infection.5,9 While a foreign object is not necessary, the presence greatly increases the likelihood of biofilm development.
Urine culture is negative but the patient responds to antimicrobial treatment.
Treatment
If a biofilm infection is suspected, treatment strategies include prompt removal of implants or foreign material in combination with appropriate antimicrobial therapy. There is no perfect treatment strategy, but options include prolonged antibiotic use (≥6 weeks [this is a highly empirical therapy unsupported by clinical evidence]), higher antibiotic dosages, and using a combination of antibiotics.6,9 Research suggests that β-lactams and aminoglycosides may help prevent the formation of a biofilm but are less useful once a colony has become established; fluoroquinolones, on the other hand, are better able to penetrate an “older” biofilm colony (ie, a well-established colony that may have secondary bacteria communities and decreased frequency of dividing and growing).1 Although there is no clinical evidence to support their effect on the biofilm, the instillation of commensal bacteria shows promise in the treatment of some infections (eg, commensal strains of Staphyloccocus epidermidis that secrete the EspA protease to prevent biofilm formation and nasal colonization by S aureus in humans). These low-virulence bacteria cause passive interference with more pathogenic strains, and this strategy has shown success in human cases of recurrent UTI and vaginitis (eg, with Lactobacillus spp).4,10
Research in human medicine is exploring potential strategies that may prove useful in eliminating these infections, including strategies to induce the dissolution of biofilms and the use of low-energy surface acoustic waves to disrupt biofilm formation. Research is under way to investigate drugs that target biofilm-specific enzymatic activity and promote dispersion signals to break up biofilms to increase susceptibility to antimicrobial therapy.1,9
Prevention
One of the most successful strategies in the fight against biofilms is aggressive prevention. Utilizing best practices when placing and maintaining indwelling urinary catheters can decrease infection rates. Indwelling catheters should be avoided when possible, and staff should be encouraged to consistently follow general recommendations to eliminate microorganism migration. These recommendations include washing hands with antiseptic soap for at least 30 seconds or using alcohol-based hand rubs between patients if water is not available.11 In addition, it is critical to avoid contamination when manipulating catheter connection sites. Catheters that become infected or soiled should be replaced immediately.5 Despite these strategies, rates of catheter-associated UTIs remain high.12
Biofilms can contribute to chronic infections, and treatment of these infections can be challenging. In the future, new treatment modalities may offer options for preventing and eliminating biofilms.
PCR = polymerase chain reaction, UTI = urinary tract infection
KELLY E. O’NEILL, DVM, will be an internist at St. Francis Veterinary Specialists in Decatur, Georgia, as of September 2014. Her professional interests include acute and chronic kidney disease and hemodialysis. She received her DVM from Colorado State University and completed a small animal internal medicine residency at Tufts University.
MARY ANNA LABATO, DVM, DACVIM (Small Animal), is clinical professor and section head in small animal medicine at Tufts University, where she also earned her DVM. She has a special interest in renal disease and interventional therapies. Dr. Labato has numerous journal publications.
LINDA ROSS, DVM, MS, DACVIM, is associate professor in small animal internal medicine at Tufts University. Her special interest is nephrology, urology, and endocrinology. She teaches courses in the pathophysiology of the urinary and endocrine systems, along with nephrology/urology and endocrinology in small animal medicine and surgery. She earned her DVM from University of Illinois.