Acute Pelvic Limb Paresis & Respiratory Effort in a Cat
Mark A. Oyama, DVM, DACVIM (Cardiology), University of Pennsylvania
Danielle S. Laughlin, DVM, DACVIM (Cardiology), BluePearl Pet Hospital, Sandy Springs, GA
Watson, a 9-year-old, castrated domestic short-haired cat, was presented for acute onset of pelvic limb paresis, increased vocalization, and progressive apparent breathing difficulties.
History
Watson’s owners returned home from work and found him dragging his pelvic limbs, vocalizing excessively, and breathing with an open mouth. He had acted normally that morning. During routine wellness evaluation 6 months before presentation, a grade II/VI systolic parasternal murmur had been noted. Results of thyroid tests had been within reference ranges.
Related Article: Feline Aortic Thromboembolism
Physical Examination
On presentation 2 hours after he was found by his owners, Watson continued vocalizing and breathing through an open mouth. Rectal temperature was 99.6°F and heart rate was 220 bpm. A grade II/VI parasternal murmur and gallop rhythm were heard on thoracic auscultation. Heart sounds were mildly muffled ventrally, and increased bronchovesicular sounds were noted bilaterally.
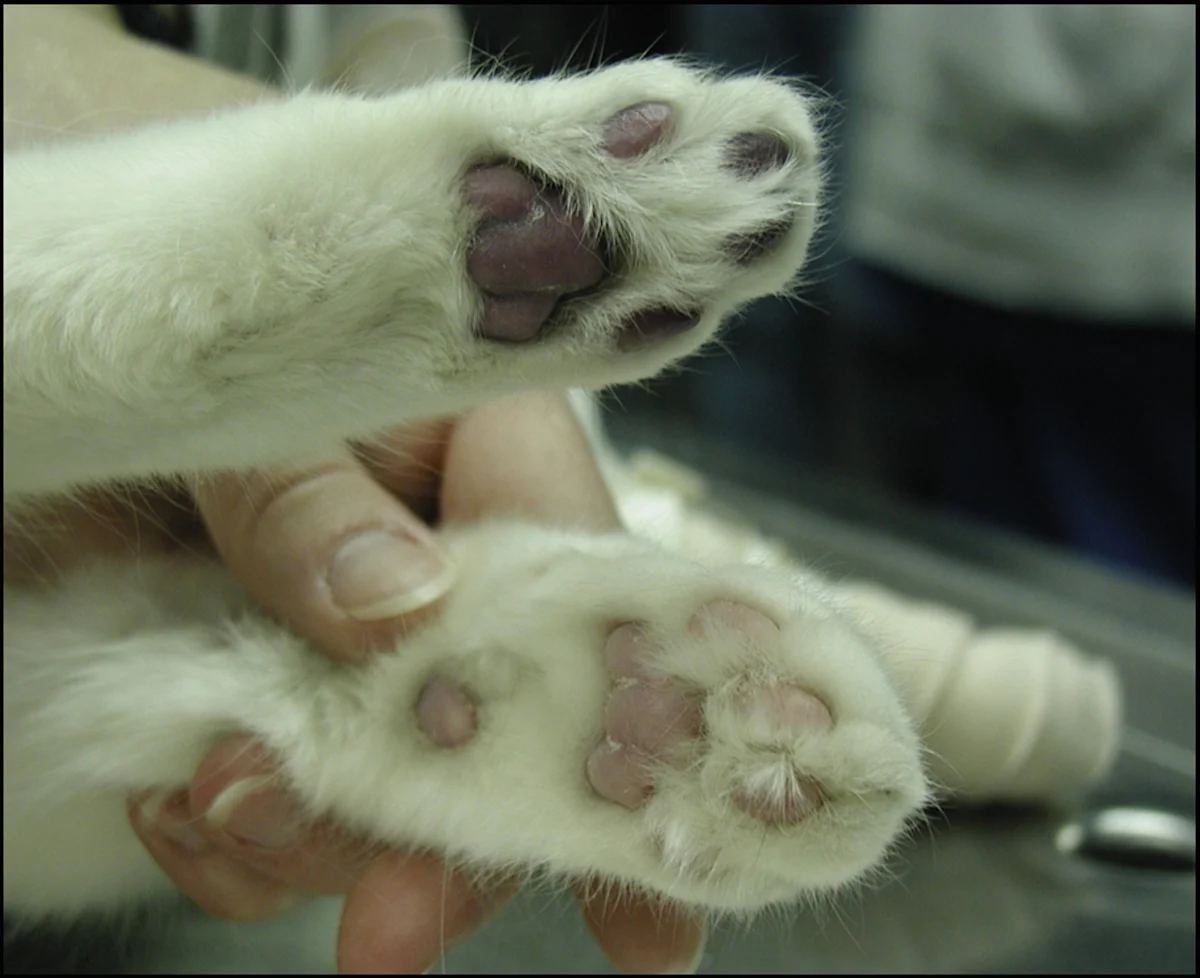
Cyanosis evident on the digital and metatarsal pads of one of the pelvic limbs at presentation (top). Normal pad from one of the cat’s thoracic limbs is shown for comparison. Image courtesy Barret Bulmer, DVM, DACVIM (Cardiology)
Swelling of the gastrocnemius muscles of the pelvic limbs was noted bilaterally; on palpation, the limbs were firm and paws were cold to the touch. The digital and metatarsal pads were markedly dark and cyanotic (Figure 1). Voluntary motor function of the pelvic limbs was absent below the hip. Deep-pain testing was negative in both pelvic limbs.
General Practice Skill: Create a Problem List
Generate an initial problem list based only on patient history and physical examination results as described above.
Being an excellent veterinary diagnostician depends on a solid foundation in the basics. Explore more problem list challenges in our General Practice Skills series here.
Imaging
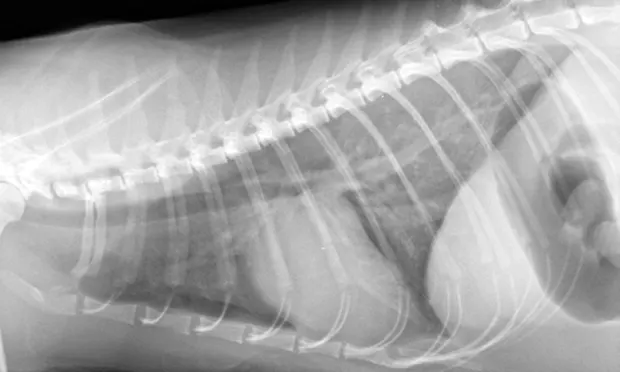
Thoracic radiography (Figure 2) revealed cardiomegaly (vertebral heart score of 8 [range, 7.2–7.8]) characterized by left atrial and ventricular enlargement, distended pulmonary veins, and a pulmonary interstitial pattern. The location of the pulmonary edema was more severe in the left lung lobe. Feline pulmonary edema may not follow the classic caudodorsal and perihilar distribution pattern noted in canine patients.
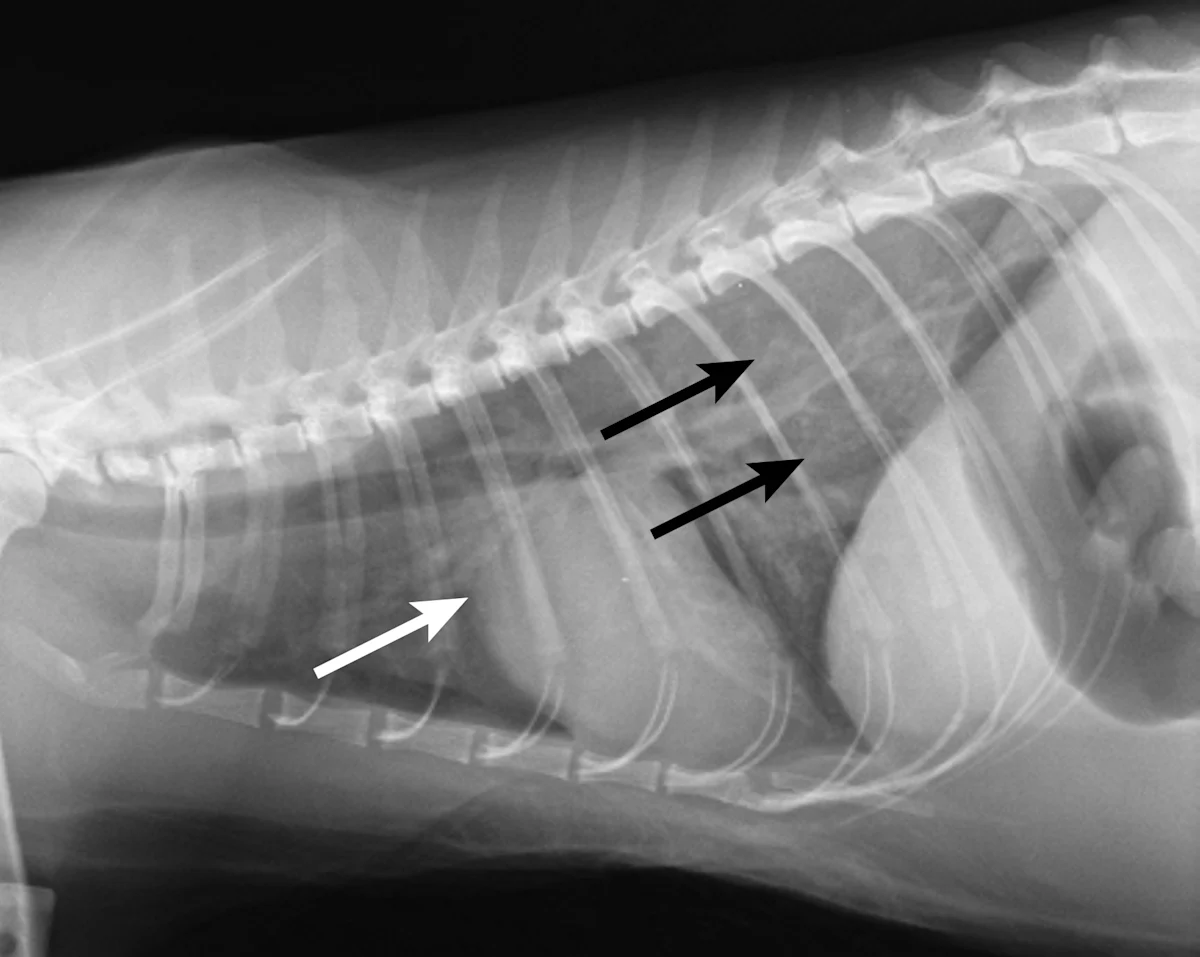
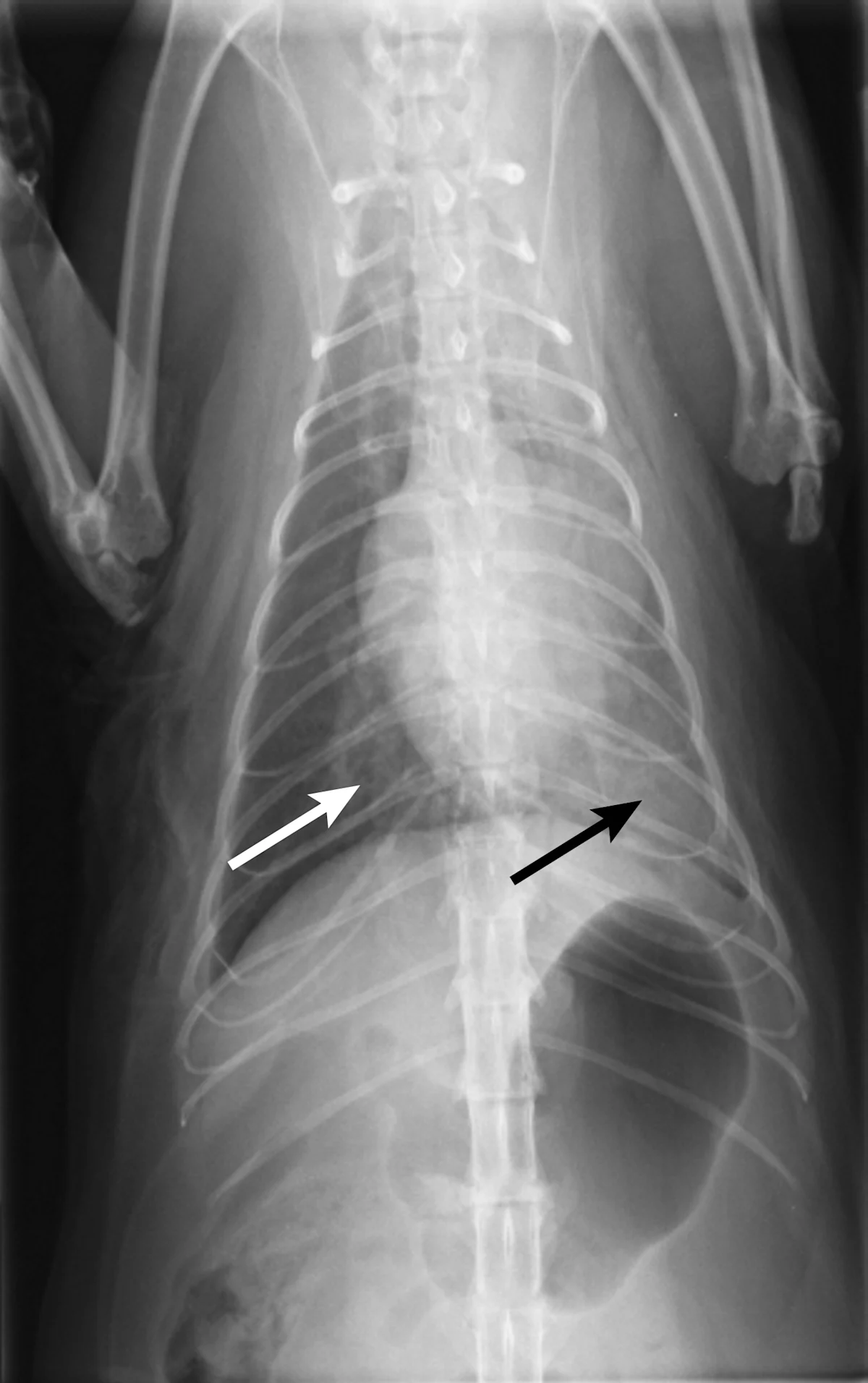
Figure 2 Right lateral (A) and dorsoventral (B) thoracic radiographs showing cardiomegaly, pulmonary venous distention (white arrows), and pulmonary interstitial densities (black arrows)
Gas distention of the stomach was evident and consistent with aerophagia secondary to pain and/or respiratory distress. Electrocardiography after initial treatment (Figure 3) demonstrated a heart rate of 150 bpm and ventricular bigeminy. Assuming standard sensitivity (1 cm/mV), the QRS complexes were tall (upper normal, 1 mV) at 1.3 mV.

Figure 3 Lead II ECG (25 mm/sec; 10 mm/mV) disclosing a heart rate of 150 bpm and ventricular bigeminy (arrows)
Ask Yourself
What characteristics of the initial presentation and examination can be combined to reach a diagnosis?
What treatment should be instituted based on initial diagnostic findings?
What role might echocardiography play in reaching a diagnosis?
What acute and long-term prognostic considerations should be discussed with the owners?
Diagnosis
Feline arterial thromboembolism and congestive heart failure (CHF)
Additional Diagnostics
Marked left atrial enlargement was present on echocardiography after stabilization and pain management (see Treatment). A formed thrombus was apparent within the left auricle (Figure 4) and spontaneous echo contrast or smoke was present within the left atrium (Figure 5). This spontaneous echo contrast is often seen with marked chamber dilation and blood stasis; although its presence is associated with an increased risk for thrombus formation, it is not a result of a formed thrombus. Thickening of the left ventricular walls was also noted (Figure 6), which is consistent with underlying hypertrophic cardiomyopathy.
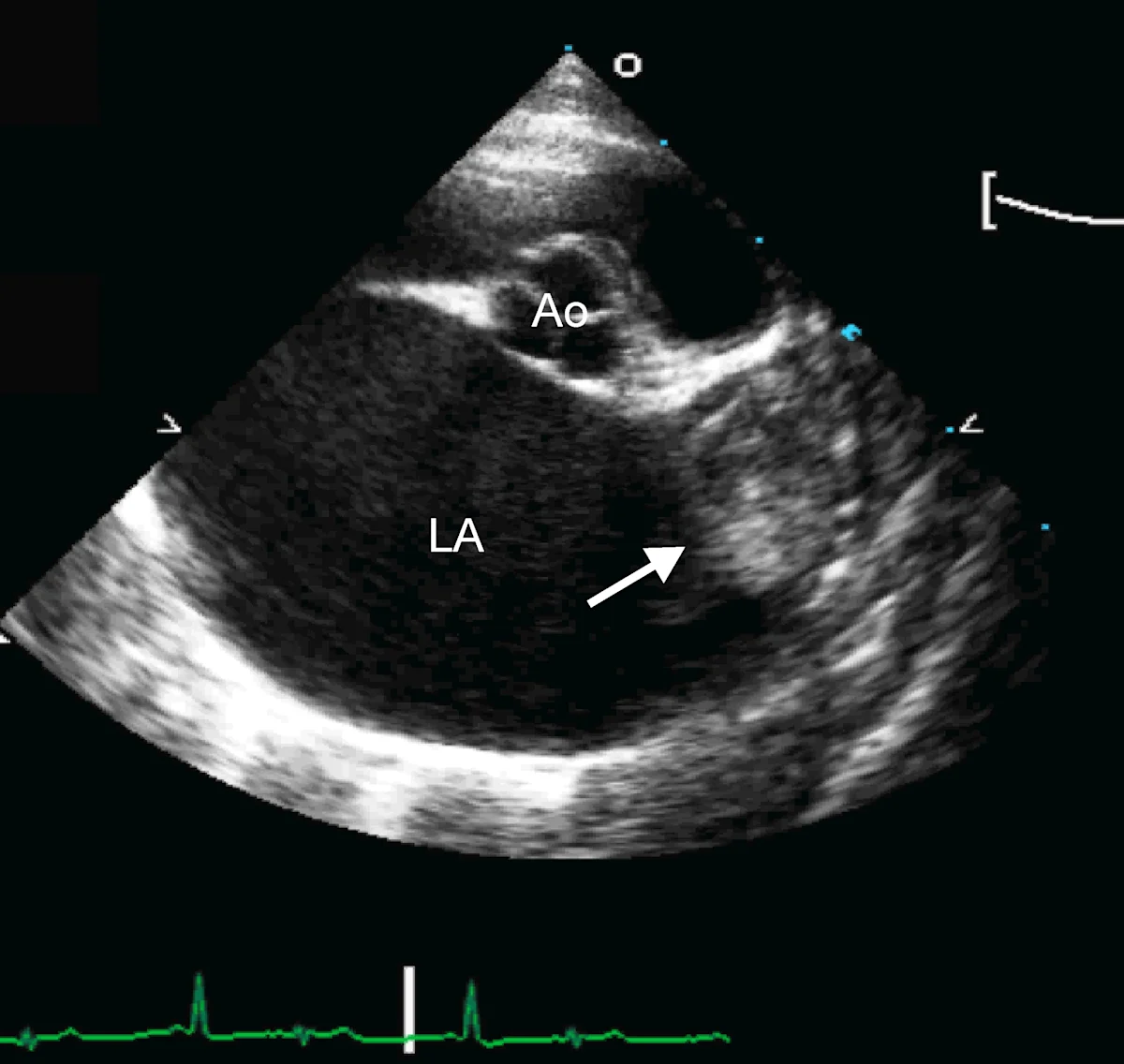
Right parasternal short-axis echocardiographic view of the heart base. The left atrium (LA) is markedly enlarged compared with the aorta (Ao). A thrombus is evident in the left auricle (arrow)
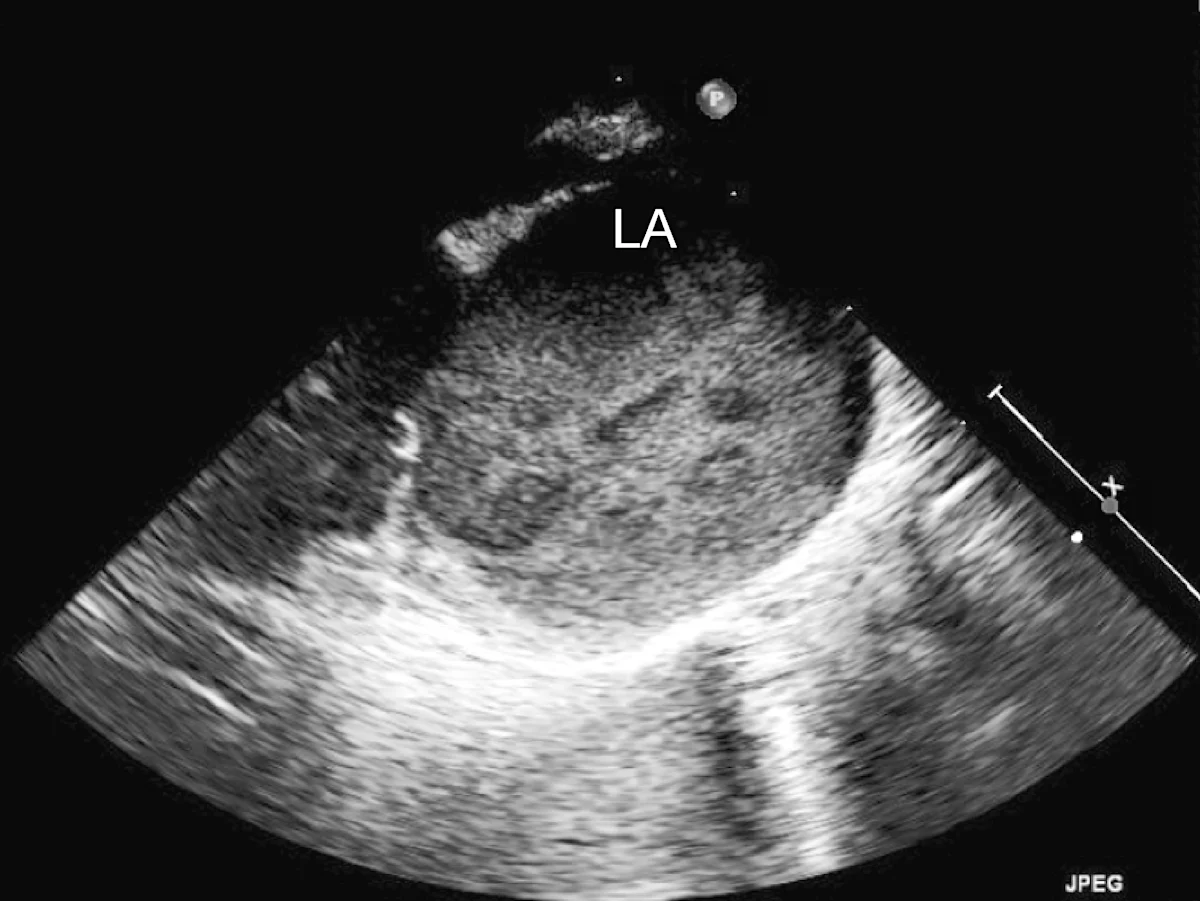
Right parasternal oblique long-axis echocardiographic view showing spontaneous contrast smoke within the left atrium (LA)
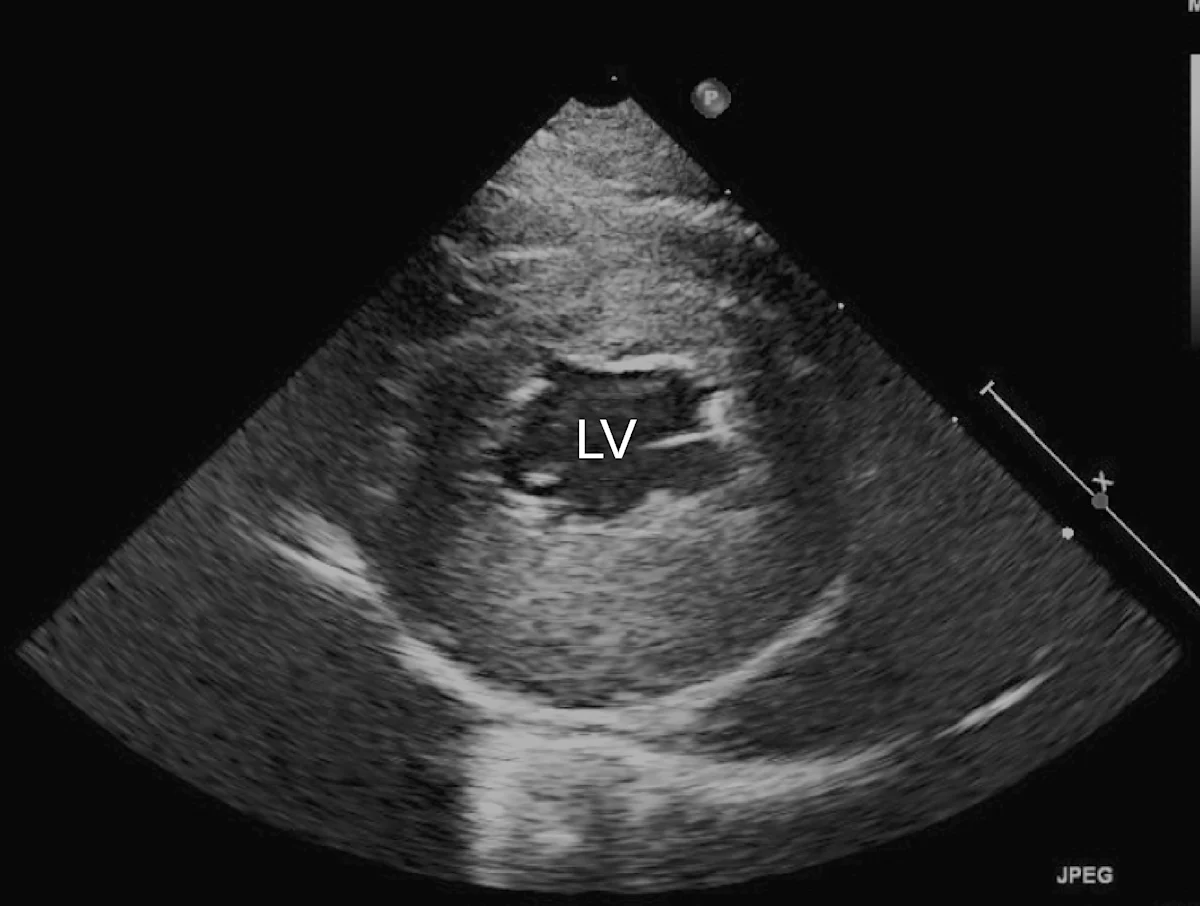
Right parasternal short-axis echocardiographic view of the left ventricle (LV) showing concentric hypertrophy, particularly of the posterior ventricular wall
A pulse could not be detected via Doppler blood pressure crystals in either limb. Results of serum biochemistry profile revealed a glucose concentration of 179 mg/dL, an alanine aminotransferase activity of 213 U/L, and an aspartate aminotransferase activity of 1113 U/L.
Treatment
At initial presentation, furosemide at 1 mg/kg IV was administered and the need for additional doses was evaluated q2h based on respiratory rate and effort. Analgesia with fentanyl (3 µg/kg/h via constant rate infusion) was initiated, and the patient was placed in an oxygen cage. After initial stabilization and pain management, unfractionated heparin was administered at a loading dose of 250 U/kg IV, followed by 200 U/kg SC q8h.
Before treatment initiation, coagulation times (PT/PTT) were monitored to assess baseline coagulation function and rule out the possibility of underlying coagulopathy (eg, disseminated intravascular coagulation associated with the thromboembolic event). PTT was monitored throughout therapy so that the anticoagulant dose could be titrated to a target PTT of 1.5 to 2 times the baseline value. Baseline serum biochemistry profile was performed before initiation of IV treatment and daily until discharge to monitor renal values and electrolytes, as elevations can occur secondary to furosemide therapy and during reperfusion.
Antiplatelet therapy included clopidogrel bisulfate at 18.75 mg PO.
There are numerous options for acute and long-term anticoagulant therapy in cats with thromboembolism, but no clear consensus exists as to the ideal treatment. Acute therapeutic options include medical therapy (eg, aspirin, unfractionated heparin, low molecular weight heparin, warfarin). Warfarin has high individual variability in cats and can lead to bleeding complications. Unfractionated heparin was used in this case because low molecular weight heparin, while purportedly providing more consistent anticoagulation, is more expensive.
Thrombolytic strategies include tissue type plasminogen activator (TPA), streptokinase, and surgical or catheter-based (rheolytic therapy) resolution of thromboemboli. TPA may be useful in cases of acute arterial thromboembolism if treatment is administered within hours of the onset of signs. Thrombolytic therapies may be difficult and might not improve survival.
Options for long-term thromboprophylaxis in cats include low-dose aspirin and clopidogrel (generally selected by the authors).
Outcome
Watson’s respiratory rate and effort rapidly improved after 2 doses of IV furosemide and initiation of oral therapy (furosemide 6.25 mg q12h, enalapril 2.5 mg q12h). Analgesics were weaned over the following 36 hours as a result of Watson’s apparent improvement and comfort. At discharge, there was no motor function in the pelvic limbs, but a faint pulse in both limbs could be detected with the Doppler blood pressure crystal.
At home, the owners confined Watson to a small area, assisted litter box access, and ensured that food and water were easily accessible. Reevaluation and repeat serum biochemistry profile 3 to 5 days later were recommended to evaluate for azotemia and electrolyte changes secondary to diuretic and angiotensin-converting enzyme (ACE) inhibitor therapy, but the owners did not return.

Medial aspect of the right pelvic limb showing necrosis and sloughing of the skin, likely from chronic ischemia of the limb
Two weeks later, Watson was presented for moist pyoderma over the pelvic limbs resulting from ischemic necrosis (Figure 7). Treatment, including wound management, was offered, but the extent of soft-tissue injury suggested poor long-term prognosis. Out of concern for Watson’s quality of life, the owners elected euthanasia.
Ask Yourself
1. What characteristics of the initial presentation and examination can be combined to reach a diagnosis?
The heart murmur, in combination with the gallop rhythm, made underlying cardiomyopathy likely. Physical examination findings consistent with arterial thromboembolism to the distal aorta or a saddle thrombus, included pain, paresis, absent pulse in pelvic limbs, hypothermia, and pallor. The cardiomegaly and pulmonary pattern seen on thoracic radiographs were consistent with concurrent left-sided heart failure.
2. What treatment should be instituted based on initial diagnostic findings?
Analgesics, furosemide, and oxygen were administered for immediate pain relief and CHF. Chest radiography helped confirm that heart failure, rather than pain, was the primary cause of increased respiratory rate and effort in this patient. Topical nitroglycerin therapy (one-eighth inch to pinna q8h) may also be initiated. Anticoagulation with heparin is common during hospitalization. Other options for acute pain management include methadone (0.2 mg/kg slow IV bolus q4–6h) and buprenorphine (0.02 mg/kg IM q6h) or morphine administered in the epidural space. In cats with marked pain, buprenorphine alone is insufficient. An alternative to clopidogrel is aspirin (81 mg PO q72h).
3. What role might echocardiography play in reaching a diagnosis?
Echocardiography helped definitively diagnose underlying cardiomyopathy and the likely origin of the thrombus. The hypertrophied left ventricular wall is consistent with hypertrophic cardiomyopathy if certain conditions (eg, hyperthyroidism, hypertension) are ruled out. The markedly enlarged left atrium and spontaneous contrast indicate high risk for thromboembolic complications. In Watson, a formed thrombus within the left auricle was detected, which further supported a diagnosis of feline arterial thromboembolism.
Although an echocardiogram helped determine the presence and severity of underlying cardiomyopathy, left atrial enlargement, and the thrombus, physical examination and radiographic findings were sufficient to achieve a presumptive diagnosis and initiate therapy with oxygen, diuretics, and antiplatelet drugs.
4. What acute and long-term prognostic considerations should be discussed with the owners?
The long-term prognosis for cats with arterial thromboembolic disease is poor. In one study of 127 cats presenting with acute aortic thromboembolism,1 overall survival rate was 35% (similar to other reported findings). Of cats that survived the initial episode and whose owners elected for hospital treatment, 45% survived to discharge. Cats with higher rectal temperature and heart rate, only one affected limb, and preserved motor function at diagnosis tended to fare better than those with hypothermia, bradycardia, and bilateral paresis/paralysis. Improved survival with only one affected limb has been reported by other investigators; this may be associated with the smaller volume of vascular bed lost to systemic circulation, less muscle mass affected by loss of perfusion, less reperfusion injury, and less release of vasoactive substances that can cause shock.
Future complications include repeated thromboembolic events, CHF, and renal dysfunction secondary to CHF treatment.
CHF = congestive heart failure, PT = prothrombin time, PTT = partial thromboplastin time, TPA = type plasminogen activator