
Treating veterinary patients, especially exotic species, with acute respiratory distress can be daunting. Although the causes of respiratory distress differ among species, basic stabilization has more similarities than differences in cats, dogs, reptiles, small mammals, and birds (Figure 1).

Basic stabilization of respiratory distress
Recognizing Respiratory Distress
Signs of Respiratory Distress in Dogs & Cats
In dogs and cats, classic signs include elevated respiratory rate with increased effort, often with an orthopneic posture that includes an outstretched neck to facilitate laminar flow of air down a straight trachea and/or abducted elbows to open the chest cavity and increase negative pressure in the thorax, drawing more air into the lungs. Cats often display open-mouthed breathing, and both cats and dogs can breathe loudly enough to be heard without a stethoscope (ie, audible breathing sounds). Cyanosis or nasal or ocular discharge may be present in some cases but is rare. True cyanosis is uncommon due to subjective differences in interpretation of mucous membrane color and the need for <85% hemoglobin saturation and >5 mg/dL deoxygenated hemoglobin volume to show a true blue color.1
Signs of Respiratory Distress in Small Mammals
In small mammals (eg, ferrets, chinchillas, guinea pigs), presentation is similar to dogs and cats. Clinical signs in obligate nasal breathers (eg, rabbits, guinea pigs, other rodents) also commonly include nasal flaring (Figure 2) and, in some cases, stertorous breathing due to partial nasal airflow obstruction.

Nasal flaring in a chinchilla with respiratory distress
Signs of Respiratory Distress in Reptiles
In reptiles, an open mouth with increased respiratory rate and effort indicates respiratory distress, not hissing or other aggressive behaviors. Orthopneic posture includes holding the head away from the body axis (eg, lizard holding the head elevated at a 45-degree angle [Figure 3], snake holding either the head above the ground or portions of the body vertically along the wall of the enclosure). Audible increased respiratory sounds (eg, stertor) and/or nasal discharge are possible, especially in lizards and tortoises. Reptiles are often anorectic.

Orthopneic posture in a leopard gecko with respiratory distress. Image courtesy of Satoshi Haginoya, BVSc
Less common clinical signs of respiratory distress include a swollen cervical region in snakes, secretions around the nose in lizards and turtles, and bubbling from the nares or scale depigmentation in turtles. Aquatic turtles may have buoyancy disorders.
Signs of Respiratory Distress in Birds
In birds, tachypnea and extension of the neck are often seen. There may also be audible inspiratory stridor or expiratory wheezes, increased respiratory effort, a cough, open-mouthed breathing, and/or tail movement with breathing suggestive of increased respiratory effort (Figure 4).

Extended neck and open-mouthed breathing in an African grey parrot with respiratory distress
Birds in respiratory distress typically appear unthrifty (Figure 5) and have decreased appetite.

Unthrifty appearance (eg, balled-up posture [not standing or perched], ruffled feathers, closed or semiclosed eyes, lethargy) in a cockatiel with respiratory distress
Administering Oxygen Therapy
Oxygen Therapy in Dogs, Cats, Small Mammals, & Birds
The priority should be to provide oxygen therapy without creating excessive stress. In most cases, flow-by oxygen therapy is fastest, provides ≥30% fraction of inspired oxygen (FiO2), and allows concurrent examination (Figure 6).
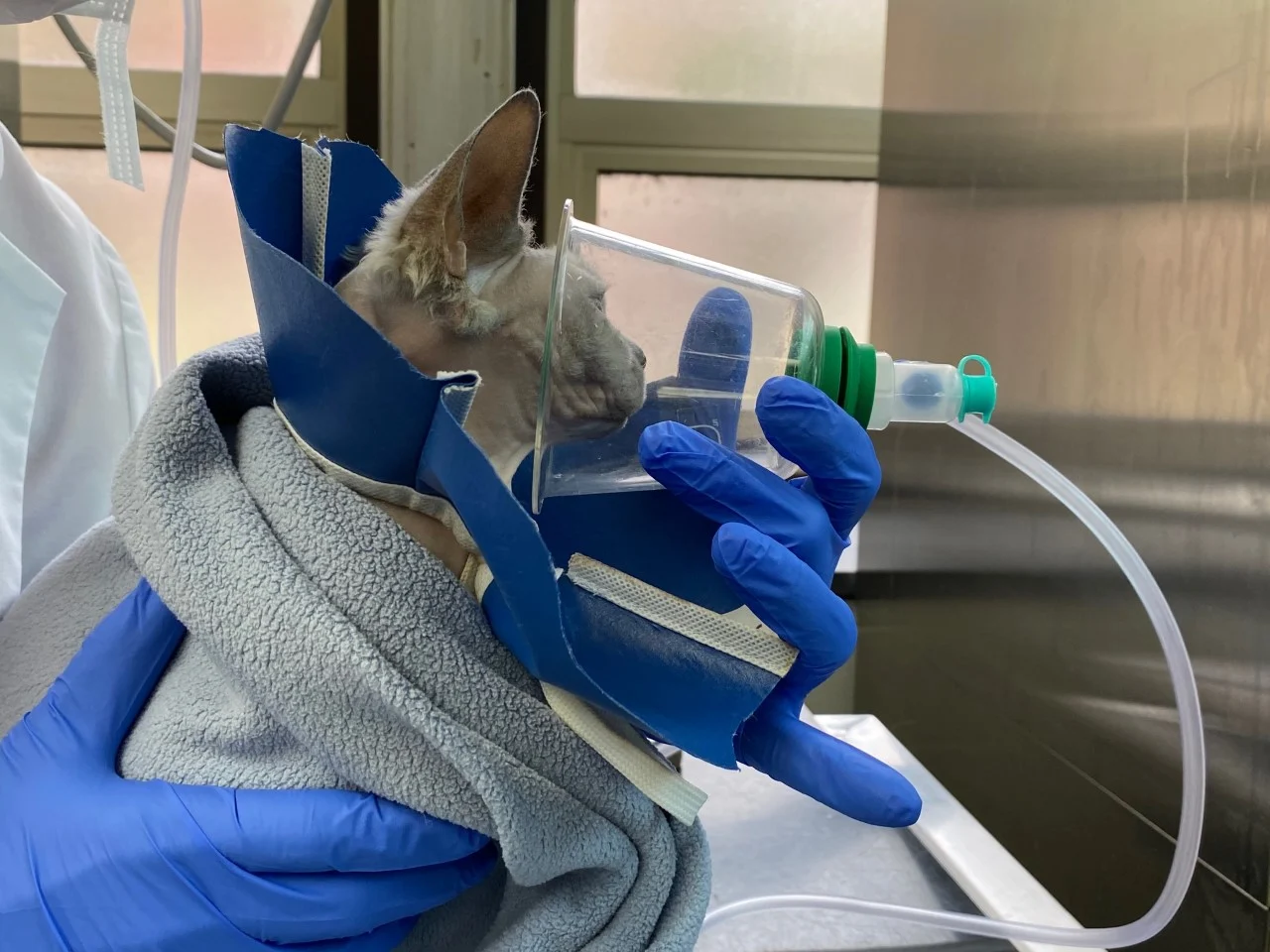
Cat receiving flow-by oxygen therapy. Image courtesy of Satoshi Haginoya, BVSc
Oxygen chambers cause minimal stress but limit the ability to examine the patient, as opening the chamber leads to loss of oxygen from the cage. In clinics that do not have a formal oxygen cage, a plastic cage door can be used to contain oxygen, and smaller incubators can be useful for exotic species, small dogs, and cats (Figure 7). Commercial cages and incubators allow oxygen settings between 21% and 60% FiO2, whereas plastic cage doors do not control FiO2 but typically reach ≥40%.

FIGURE 7 Commercial oxygen cage (A), plastic cage door replacement to contain oxygen (B), and incubator with oxygen (C). Images courtesy of Satoshi Haginoya, BVSc
If a true oxygen cage is not available, oxygen can be provided temporarily by placing a cat or small dog carrier in a plastic bag filled with oxygen, using a plastic box (eg, anesthetic induction box) as an oxygen cage, covering the bottom 75% to 80% of an Elizabethan collar with plastic wrap to contain oxygen, or wrapping a cage door with plastic to create an oxygen chamber (Figure 8). All oxygen chambers should have a hole to admit oxygen tubing and an exhaust hole for carbon dioxide and heat to pass through.

FIGURE 8 Small carrier in a plastic bag filled with oxygen (A) and a plastic box (B) used as alternative oxygen cages. A hole is present in the back of the bag and the side or back of the box to allow exhaust of carbon dioxide and heat.
Oxygen cages can quickly become hot and/or humid, especially if a patient is large relative to the size of the cage or a canine patient is panting. The temperature should be checked regularly by inserting a thermometer or opening the door wide enough to insert a hand to test the temperature and humidity. Oxygen levels drop quickly when an oxygen cage door is opened. If regular patient handling is required, an oxygen cage is not an efficient way to deliver consistent oxygen.
Intranasal cannulas can provide oxygen to dogs, usually during long-term hospitalization, and allow for patient handling during oxygen administration. Single nasal cannulas can provide ≈30% FiO2, and bilateral cannulas can provide 40% to 50% FiO2. Cannulas are not commonly used in patients smaller than dogs, as available tubes are too large for the nostrils, and stress associated with placement of cannulas may be too high in cats and exotic species.
Oxygen Therapy in Reptiles
In reptiles, respiration is driven primarily by hypoxia (compared with hypercapnia in mammals and birds) and decreases with lowered temperatures.2,3 Oxygen therapy (typically, flow-by or oxygen cage/incubator) should therefore be performed cautiously; low levels (≤30%-40% FiO2) should be administered to avoid reducing the ventilatory drive.4 Intubation with positive-pressure ventilation is needed to effectively deliver higher levels of oxygen in patients not responding to treatment.2,3 As ventilation in reptiles is decreased by hypothermia, distressed patients should be kept warm with an incubator, hot water bottles, or another similar technique (Figure 9). Species-specific preferred optimum temperature zones are described in the literature and should be followed during hospitalization.3

Leopard gecko given flow-by oxygen and heat support via warmed water in a glove. Image courtesy of Satoshi Haginoya, BVSc
Categorizing Respiratory Distress
Following oxygen administration, respiratory distress should be categorized as upper or lower airway disease to help determine next steps for stabilization. There can be overlap between these 2 categories if a patient has concurrent upper and lower airway disease.
Upper Airway Disease
Patients with upper airway disease have loud breathing (ie, stertor, stridor), inspiratory dyspnea, and clinical signs of anxiety (eg, staring with eyes opened widely, pacing, not sitting quietly) and may be cyanotic.5,6 In all species, upper airway disease is located in the nasal, oropharyngeal, laryngeal, or cervical tracheal region and can range from neoplastic masses, abscesses, and other space-occupying lesions to inflammation obstructing airflow. Cats, reptiles, and birds often have viral, bacterial, or parasitic upper respiratory infections that cause upper airway signs of varying severity.
Emergency Stabilization of Upper Airway Disease
Upper airway disease is acutely treated by following the mnemonic NOSE: Noisy breathing means Oxygen, Sedation, and Endotracheal tube placement (ie, intubation) if needed.
Oxygen Administration
Oxygen should be administered in the least stressful manner possible, and patients should be handled as little as possible to reduce stress.
Sedation
Sedation can be provided in all species by administering butorphanol (0.1-0.5 mg/kg IV or IM) and/or acepromazine (0.01-0.5 mg/kg IV or IM). Acepromazine should be administered at the high end of the dose range in most exotic species and at the low end (typically) in dogs and cats. Specific butorphanol and acepromazine doses can be found in species-appropriate formularies. Butorphanol has a rapid onset of activity, but acepromazine can take up to 20 to 30 minutes to reach full effect, especially when given IM, and should not be readministered prematurely.
Exotic species may benefit from sedation protocols that include an opioid and a benzodiazepine, rather than an opioid alone, as this combination can provide additional stress relief and tranquilization/sedative effects and increase tolerance of manipulation. In addition, most benzodiazepines can be reversed with flumazenil, and most opioids can be reversed with naloxone during a crisis. Sedation in exotic species should be considered on a case-by-case basis depending on the species and clinical condition. Some species (eg, reptiles) may have higher sensitivity to sedation and prolonged drug clearance. A formulary should be consulted for species-specific doses.
Endotracheal Tube Placement
Intubation may be needed if a patient’s condition is declining or does not improve after 10 to 15 minutes of oxygen therapy and sedation or if the patient is a reptile breathing fewer breaths per minute after oxygen therapy compared with before (ie, oxygen is suppressing the ventilatory drive). In the authors’ experience, propofol IV or alfaxalone IV or IM to effect is often the most accessible induction medication and readily allows for endotracheal intubation.
Following intubation, repeated administration of propofol, alfaxalone, or another injectable anesthetic drug can be performed as needed to keep the patient relaxed and comfortable. Repeated doses should be small—just enough to keep the patient tolerant of the endotracheal tube and handling. Inhalant anesthetics should be avoided due to the risk for hypotension and respiratory depression. Patients should remain intubated until the cause of upper respiratory distress is identified and/or treated.
Oxygen therapy during intubation is often not required (and is not recommended in reptiles), as establishing an airway with the endotracheal tube relieves upper airway distress. A flow-by oxygen tube or oxygen from an anesthesia circuit can be held adjacent (without attaching) to the end of the endotracheal tube if necessary. If the patient becomes dyspneic immediately or shortly after extubation (even with concurrent administration of acepromazine, butorphanol, or other sedatives), reintubation with heavy sedation or anesthesia may be required until definitive diagnostics and treatment are complete.
In birds, if intubation is not possible (usually due to tracheal obstruction), the air sacs (extensions from the lungs) can be percutaneously cannulated with a small endotracheal tube with the patient under anesthesia so oxygen can be administered directly into the air sac. Detailed descriptions of this technique are available.4
Lower Airway Disease
Lower airway disease is commonly associated with an increased respiratory rate and a cough in some species (ie, dogs, birds, small mammals).4 Patients may also have open- or closed-mouth breathing with or without markedly increased respiratory effort. Lower airway disease in small animals and mammals is localized to the lungs and pleural space. Birds and reptiles lack a diaphragm; lungs share the coelomic space with other organs (eg, liver, GI tract). Space-occupying abdominal disease (eg, free abdominal fluid, reproductive diseases, organomegaly, neoplasia) restricts expansion of the lungs and can therefore lead to lower respiratory-related respiratory distress in those species. In addition, pathology of the air sacs in birds can cause or contribute to respiratory distress.
Auscultation of the thorax is important in patients with lower respiratory disease (except in species [eg, reptiles] in which auscultation is challenging), as it allows patients to be identified as those with loud or quiet auscultation. In contrast, thoracic auscultation is difficult in patients with upper respiratory disease due to significant referred upper airway noise.
Loud Auscultation
Patients with loud thoracic auscultation (ie, crackles, wheezes) heard via stethoscope have pulmonary parenchymal or other lower airway disease. The most common diseases vary depending on species but include pulmonary edema (cardiogenic or noncardiogenic), pneumonia (bacterial, viral, or fungal), and inflammatory diseases (eg, feline asthma, parasitic lung diseases).
Emergency Stabilization of Loud Auscultation
Pulmonary parenchymal and other lower airway diseases are clinically differentiated based on radiographic pattern and location, but radiography should not be performed before dyspneic patients are stabilized. In dogs, point-of-care ultrasonography performed by trained professionals can help differentiate congestive heart failure from other parenchymal lung disease and determine whether a patient with a heart murmur has congestive heart failure7,8; however, thoracic ultrasonography may cause too much stress prior to stabilization.
Oxygen, a single dose of furosemide and butorphanol, and possibly a single dose of a bronchodilator can help emergently alleviate respiratory distress so radiography or ultrasonography can be performed to help guide diagnosis and further treatment. In some cases, and in the absence of contraindications (eg, suspected heart disease), a single anti-inflammatory dose of a corticosteroid may also be beneficial. In exotic species and cats, some medications can be administered via inhalation. In all species, patients with respiratory distress should be handled with caution to avoid inducing stress. Common lower airway disease differentials and first-line treatment options for stabilization are included in the Table.
Table: Emergency Treatments for Common Causes of Lower Respiratory Disease Characterized by Loud Lung Sounds
Quiet Auscultation
In dogs, cats, and small mammals with lower airway disease and quiet auscultation, pneumothorax and pleural effusion are the most likely differentials. Coughing and/or retching are sometimes noted in dogs and cats with pneumothorax. In reptiles and birds, respiratory distress may be due to ascites in the coelom. Reptiles are rarely auscultated due to their scales, but presence of coelomic fluid in birds can make auscultation of the air sacs and lungs challenging. Air in the coelom is uncommon. If a patient is presented with dramatic respiratory distress, the chest or coelom should be tapped immediately to remove air or fluid. In general, thoracocentesis or coelomocentesis should be used to remove as much air or fluid as possible to stabilize the patient. A tap concurrently determines whether distress is due to air or fluid and what type of fluid is present in the thorax or coelom. There is minimal danger if thoracocentesis does not yield air or fluid (ie, negative tap) in dyspneic patients.
In dyspneic patients, thoracocentesis or coelomocentesis should be performed before radiography. In minimally affected patients, radiography can be performed first. Point-of-care thoracic ultrasonography can confirm the presence of fluid or air with less stress compared with radiography in dogs and cats but should not replace immediate thoracocentesis in severely affected patients.9,10 Similarly, point-of-care lung ultrasonography can identify coelomic fluid in reptiles and birds (Figure 10) but should not replace coelomocentesis in severely affected patients.11,12

Point-of-care lung ultrasonography in a leopard gecko. Image courtesy of Satoshi Haginoya, BVSc
Emergency Stabilization of Quiet Auscultation
Patients with pleural effusion, pneumothorax, or coelomic fluid require therapeutic thoracocentesis or coelomocentesis for stabilization. Centesis can be performed blindly or via ultrasound guidance and may require the patient to be sedated (eg, with butorphanol [0.1-0.5 mg/kg IV or IM, based on species] with or without other drugs [eg, benzodiazepines]). Careful handling is needed to avoid further patient stress. If pneumothorax is continuous (ie, negative pressure indicating completion of thoracocentesis is not achieved due to continuous air leakage), a thoracostomy tube may be required for continued air removal.
Conclusion
Stabilization of patients with respiratory distress requires a calm, sequential approach. Further treatments and diagnostics are typically required to determine the exact cause of distress; however, basic stabilization can provide time to investigate common differentials (especially in species with which the clinician is less familiar) and make informed diagnostic and treatment decisions.