Ovariohysterectomy in the Teacup Pig
Daniel E. Lewer, DVM
Steven Brown, DVM
Teacup miniature pigs usually weigh between 29 lb and 39 lb and live 12 to 17 years. Although teacup pigs are not necessarily new to veterinarians, their increased popularity has created a greater demand for primary care, including ovariohysterectomy and castration procedures.
Gaining a familiarity with the anatomy and endocrinology of this species before surgery is important. For example, when compared to the canine uterus, the reproductive tract is extremely friable and is smaller in proportion to the pig that isn’t gravid. Porcine anesthesia is complicated because of limited accessible veins, difficult intubation, complicated restraint, and potential for malignant hyperthermia. With appropriate presurgical examination and blood work, teacup pigs can do well under anesthesia and recover well. Complications (eg, hypothermia, hypoglycemia) can occur if surgery takes an extended period of time (>25 min) and are similar to those of a 2-lb to 3-lb puppy undergoing a prolonged abdominal procedure.
Related Article: Cesarean Section in the Dog
Step-by-Step: Ovariohysterectomy in the Teacup Pig
Step 1
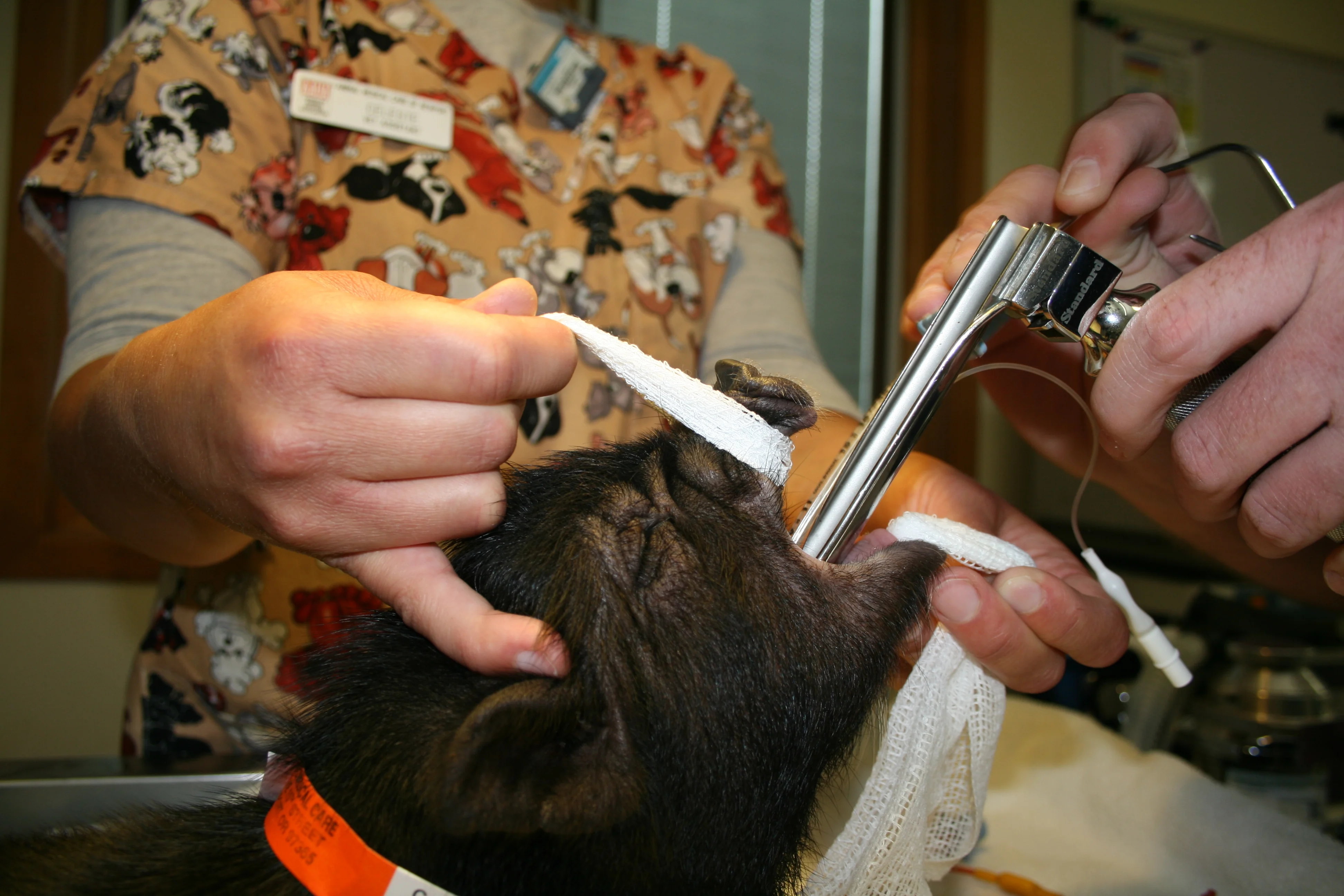
Anesthetize the patient with an IM injection of 10–12 mg/kg ketamine and 2–4 mg/kg diazepam; it takes approximately 4 min to 8 min to reach appropriate sedation for intubation. Administer 1 mg/lb carprofen (Rimadyl, pfizerah.com) intraoperatively for postsurgical pain management. (A) Place gauze around the mandible and maxilla to hold the mouth open while elongating the neck 45° to the table to prevent impinging the airway. Intubate using a size 2-3 laryngoscope (larger than that used for a dog of similar size) and a topical application of lidocaine for vocal cords. (B) Use an endotracheal tube (size ~2.5–3) with a stylet. Once intubated, keep patient on oxygen at 1000 mL/min and isoflurane 1.5%–2%.
Author Insight
Opening the mouth and elongating the neck 45° to the table is important, as pointing the throat vertically impinges the airway.
Step 2
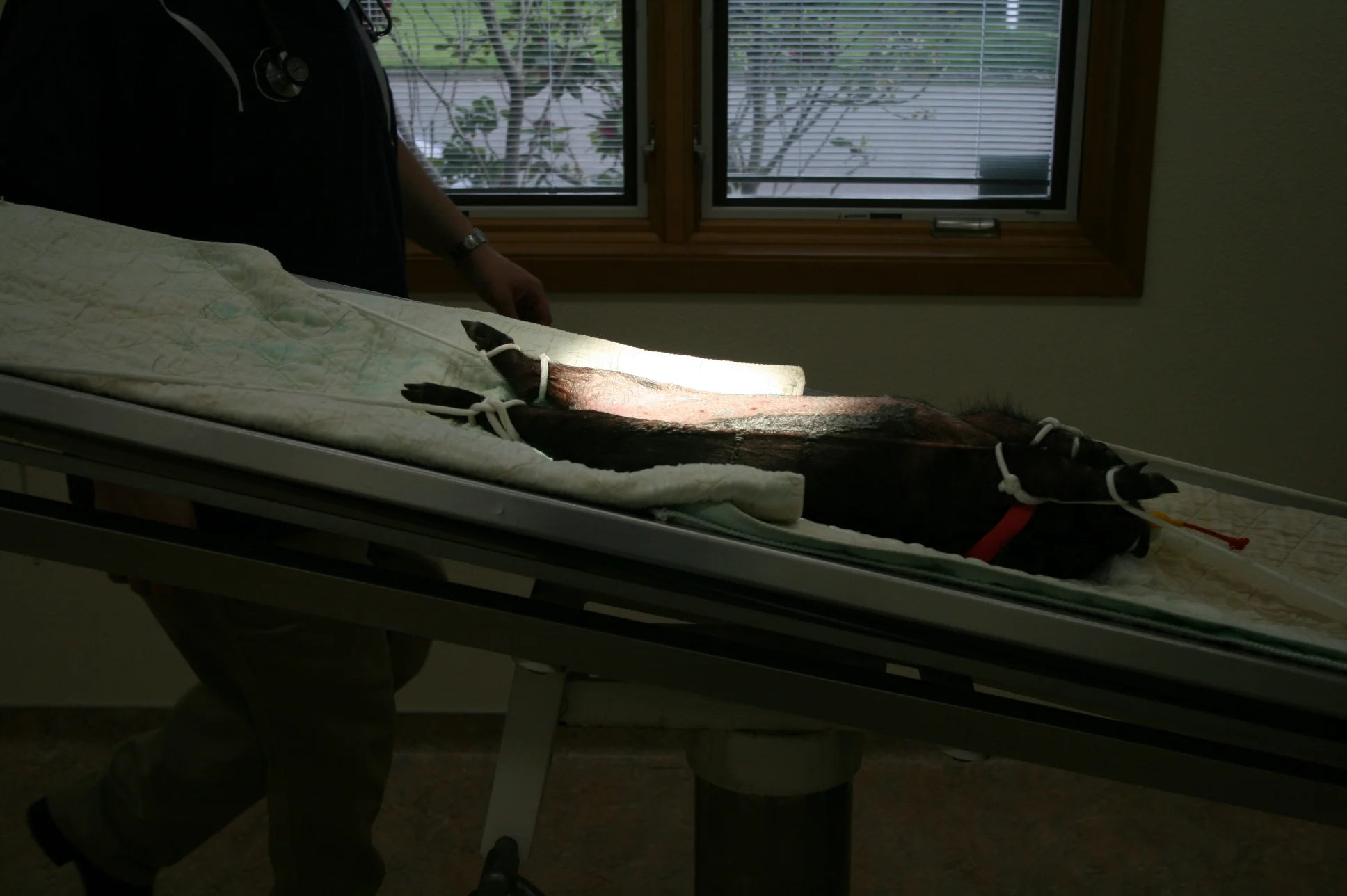
When the patient is in dorsal recumbency, the reproductive tract is pushed caudodorsally in the abdomen and covered by the bladder. Position patient in dorsal recumbency with the head at a 30° downward angle; this pulls the intestines cranially and keeps them out of the way, expediting the procedure and reducing the chance of lacerating the bowel.
Step 3
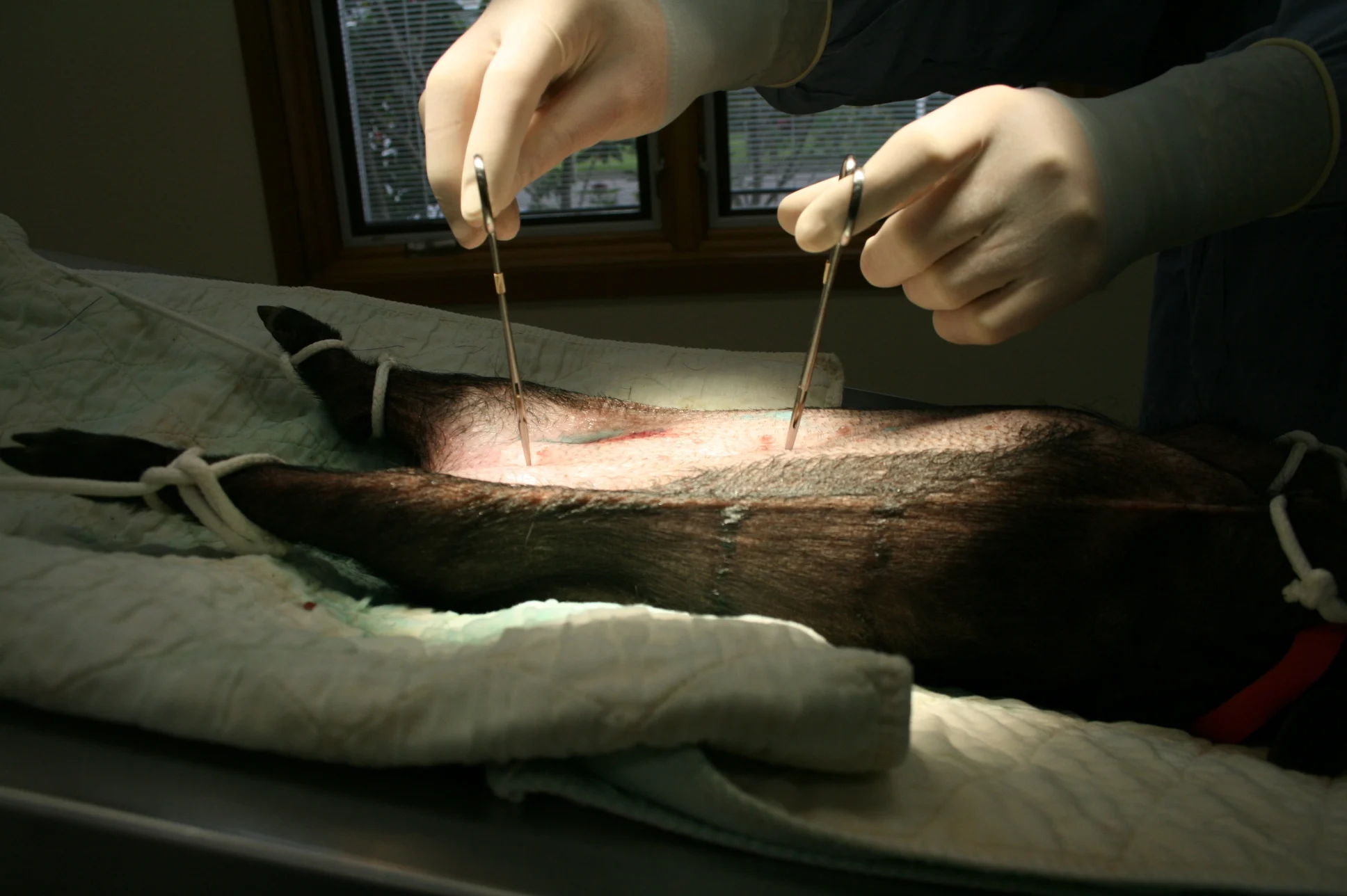
(A) Using the xyphoid and pubis as abdominal landmarks, make the initial incision in the caudal most fourth to eighth of the abdomen and extend caudally to the pubis making a 3–4 cm incision. It is a similar incision site as to where one would start for a cystostomy in a cat (B).
Bluntly dissect away fat 1 cm to 1.5 cm laterally to visualize the linea alba and assist closure. Carefully enter the peritoneal cavity with blunt Metzenbaum scissors while tenting linea away from internal organs (ie, bowel, bladder). (C) Retract linea and skin with Allis tissue forceps.
Author Insight
Use caution—the bowel is directly adjacent to the abdominal wall, making the risk for laceration relatively high.
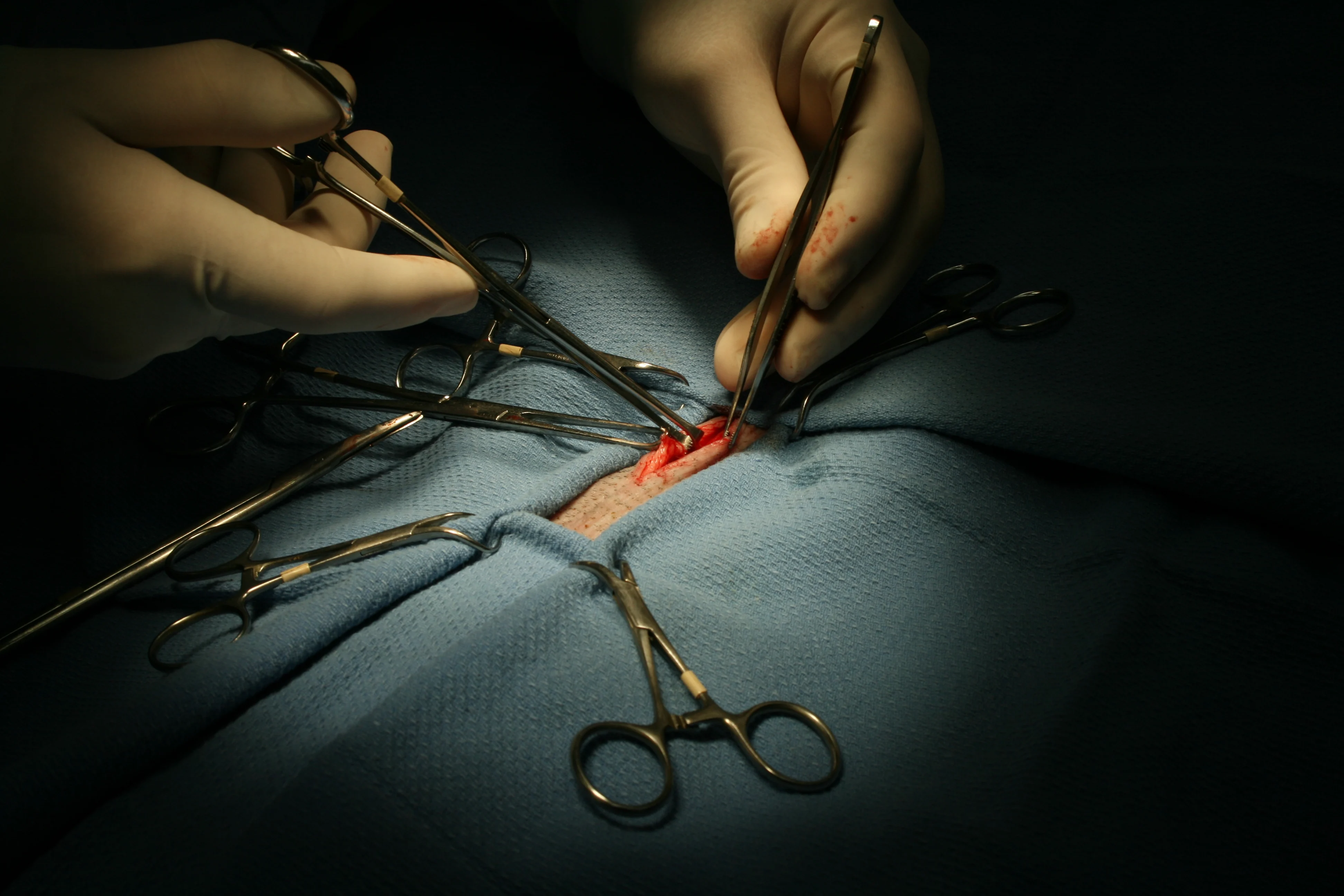
Step 4
To prevent the bladder from obscuring the surgical field, either exteriorize and caudally retract it with stay sutures or drain it via cystocentesis. The authors prefer retraction as the bladder and uterine tissue may appear similar.
Author Insight
Place a wet gauze sponge over the protruding intestines to cover and isolate them during the exteriorization of the reproductive tract.
Step 5
Use tissue forceps to hold linea and skin. The uterus, uterine horns, and ovaries may be identified dorsal to or underneath the bladder (if in dorsal recumbency). (A) Gently exteriorize and caudally retract the uterine horn. (B, C, D) Completely exteriorize and retract the ovaries and uterus down to the bifurcation, all of which can be exposed within the space of a quarter’s diameter.
Author Insight
The uterus, ovarian horns, and broad ligament are considerably more localized because they are tortuous (as opposed to elongated), friable, and vascular, making reproductive surgical technique significantly different than that on cats or dogs.
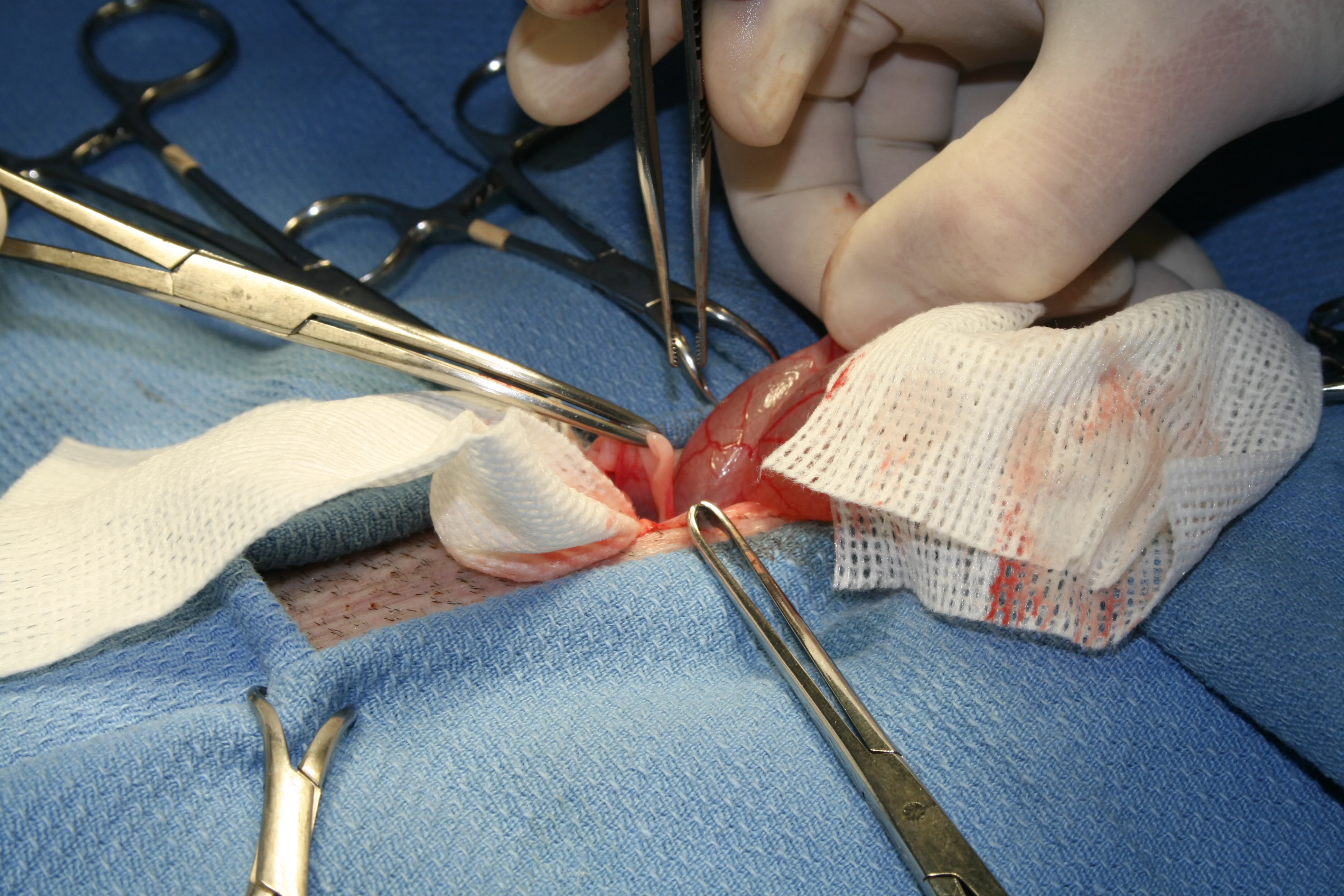
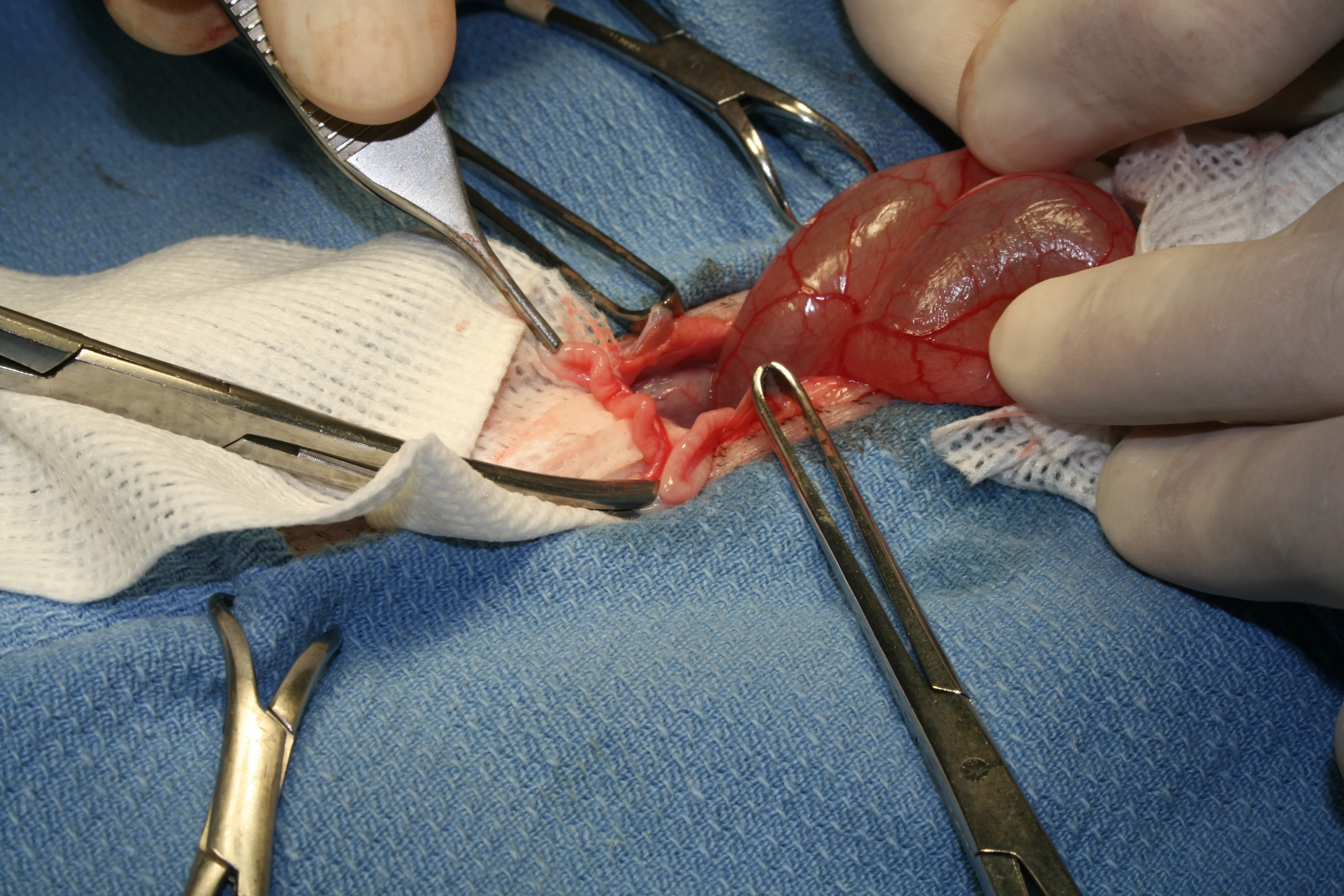
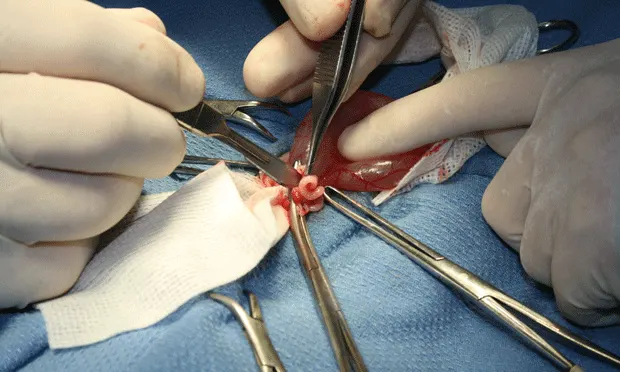
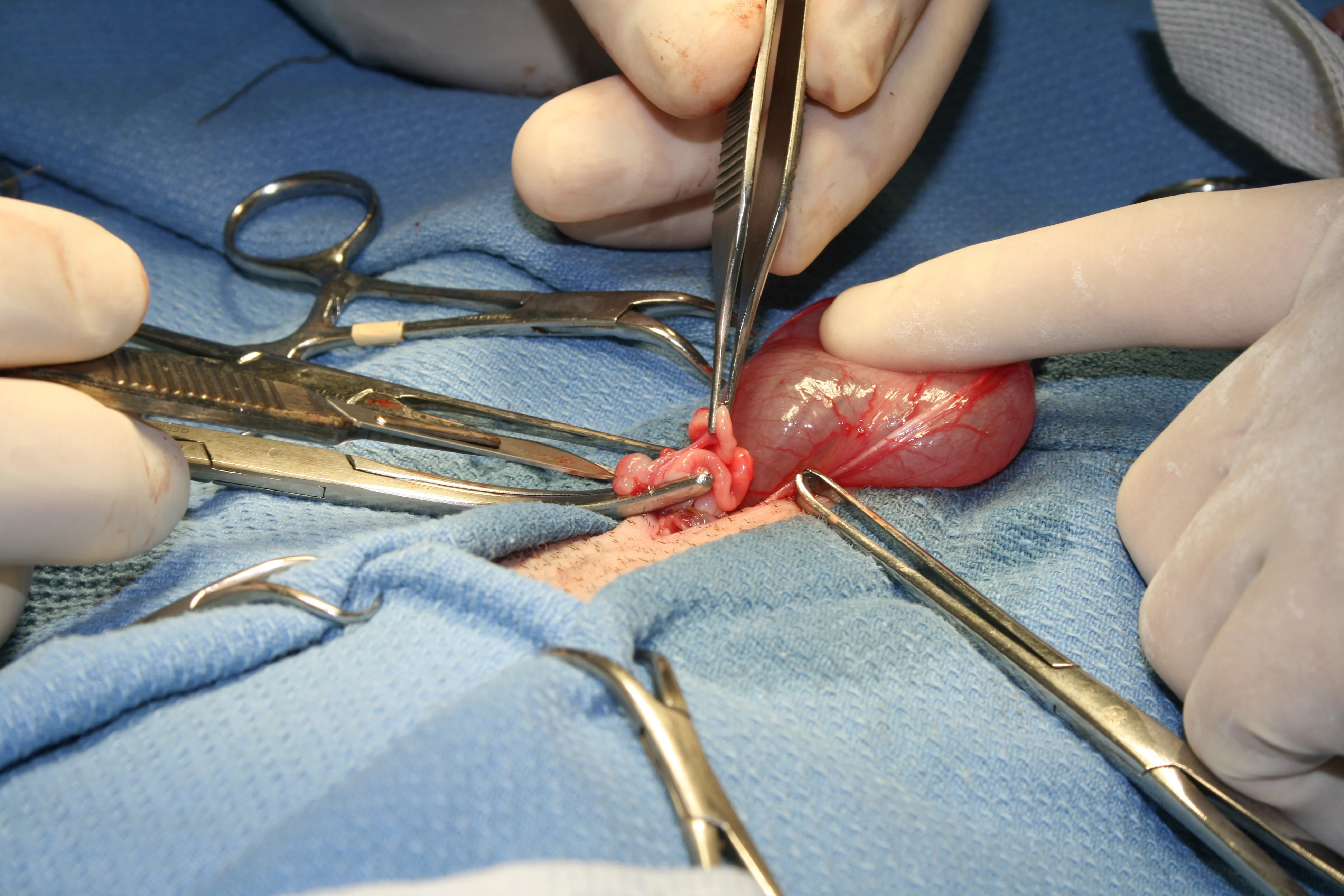
Step 6
(6A) Place initial clamp across the vascular supply to the ovary and uterine horn at the level of bifurcation, occluding the largest vasculature. (B) Using 3-0 PDS place 1 ligature proximal to the clamp and 2 at the level of uterine bifurcation. (C) Excise the ovary and horn, and the uterus to the level of bifurcation.
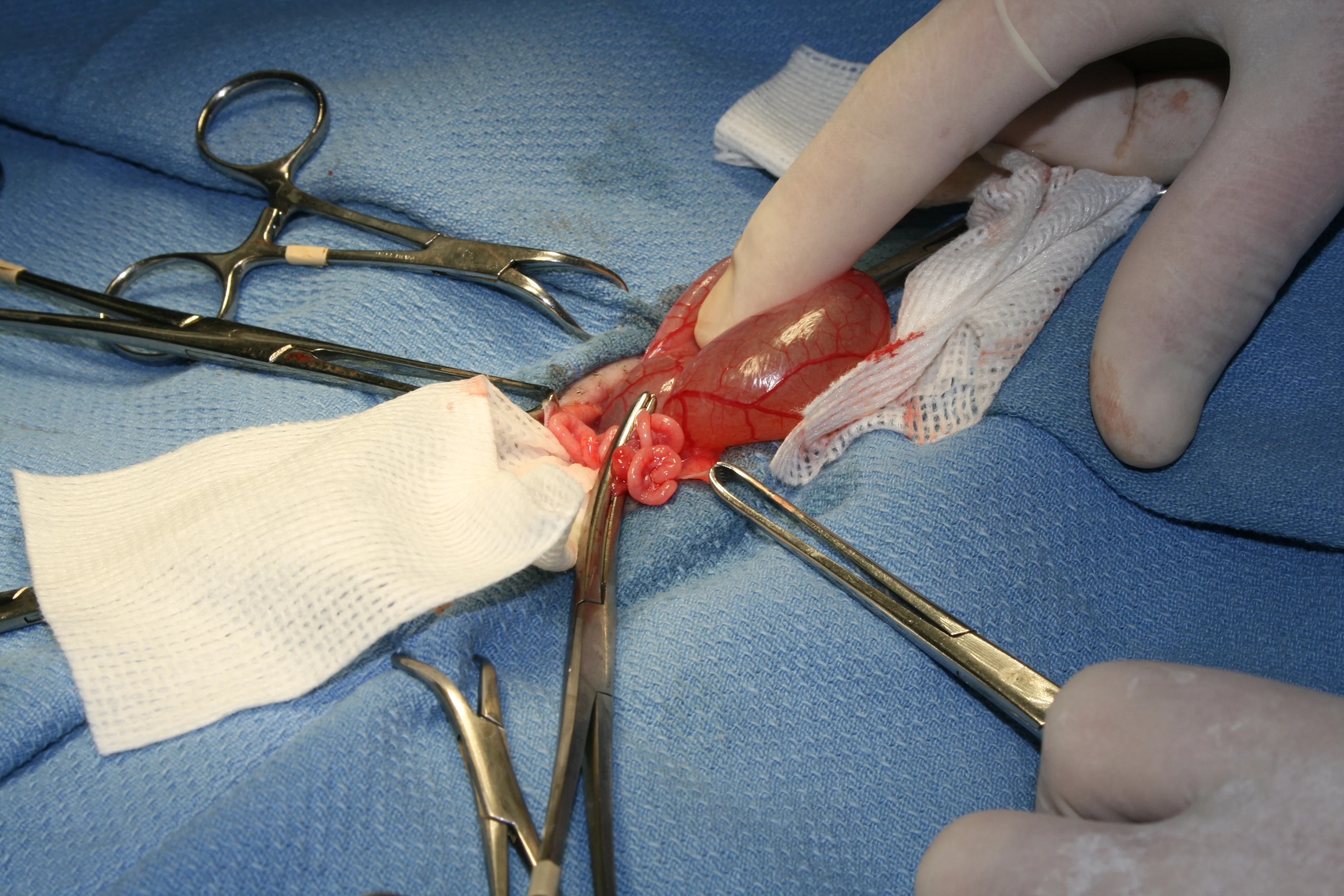
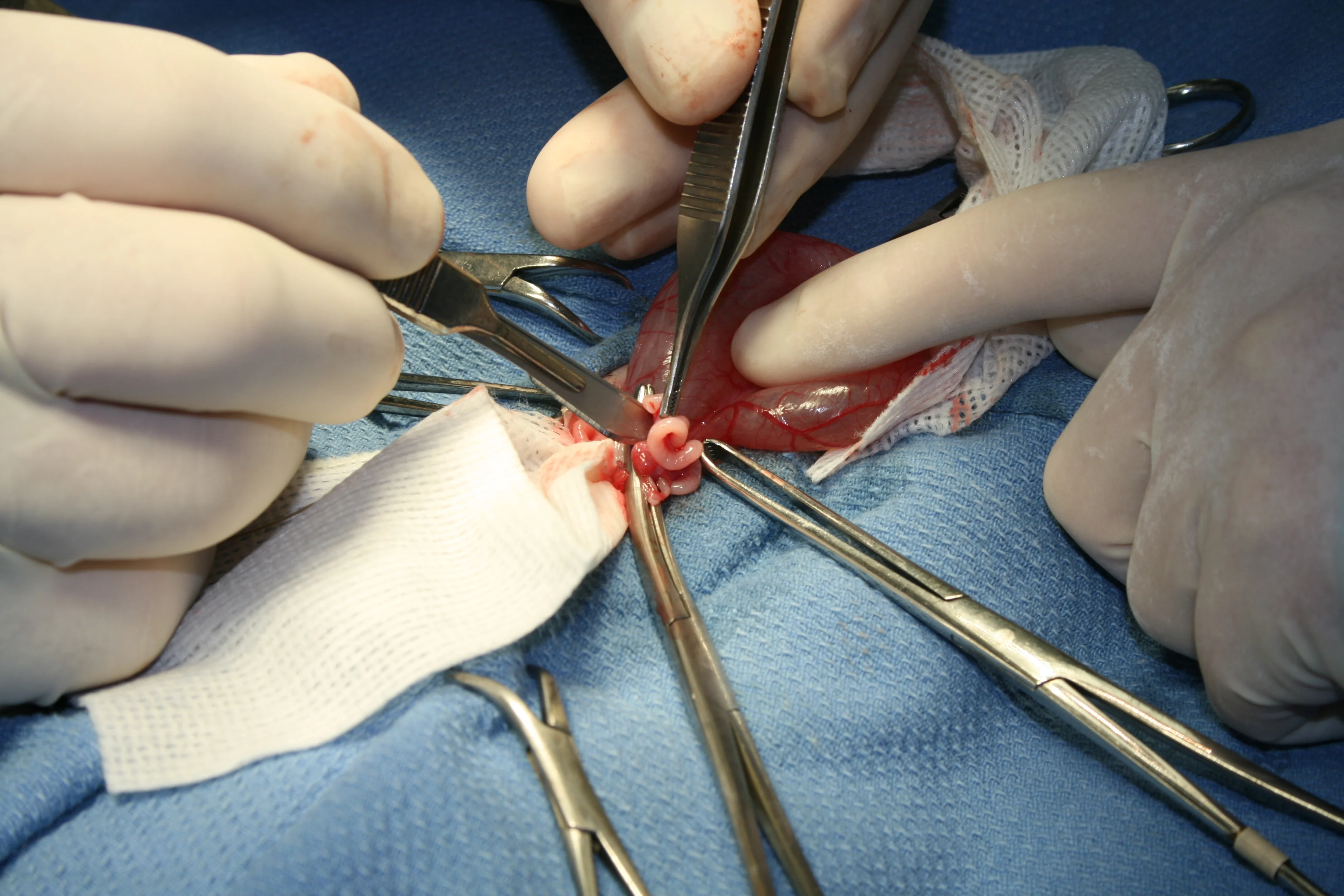
Step 7
(A) Bring the ovary and remaining uterine horn over the uterus and clamp together (B) such that blood supply for all structures are within one clamp. Ligate proximal to the clamp and then in the crush of the clamp. Transect the ovary and uterine horn distal to the clamp, check for hemostasis, and then place back into the abdomen. Check all tissue for hemostasis.
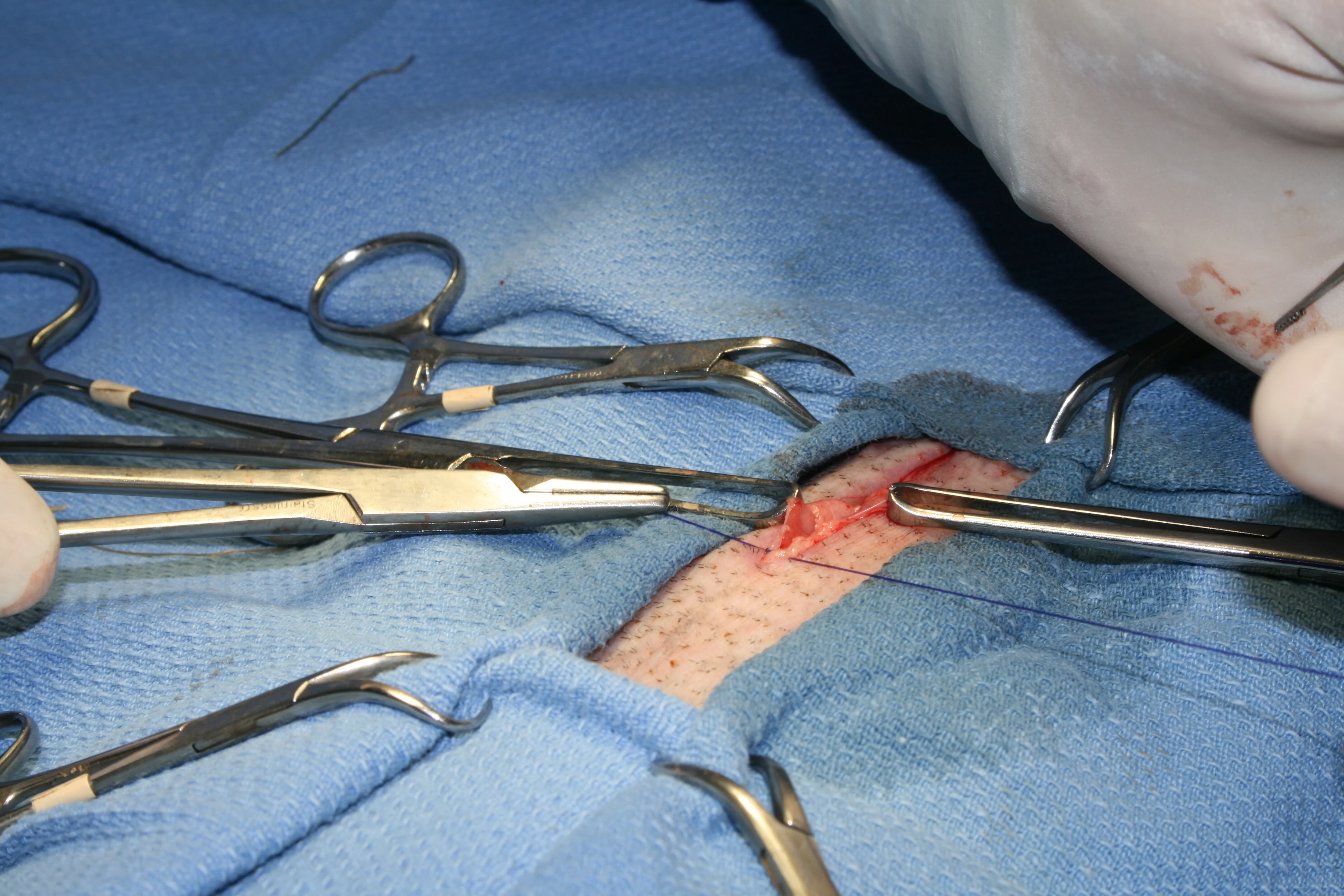
Step 8
(A and B) Close the linea albea and then the subcutaneous level using 3-0 PDS in a simple continuous pattern. (C and D) Close the skin with an intradermal continuous pattern or tissue glue.
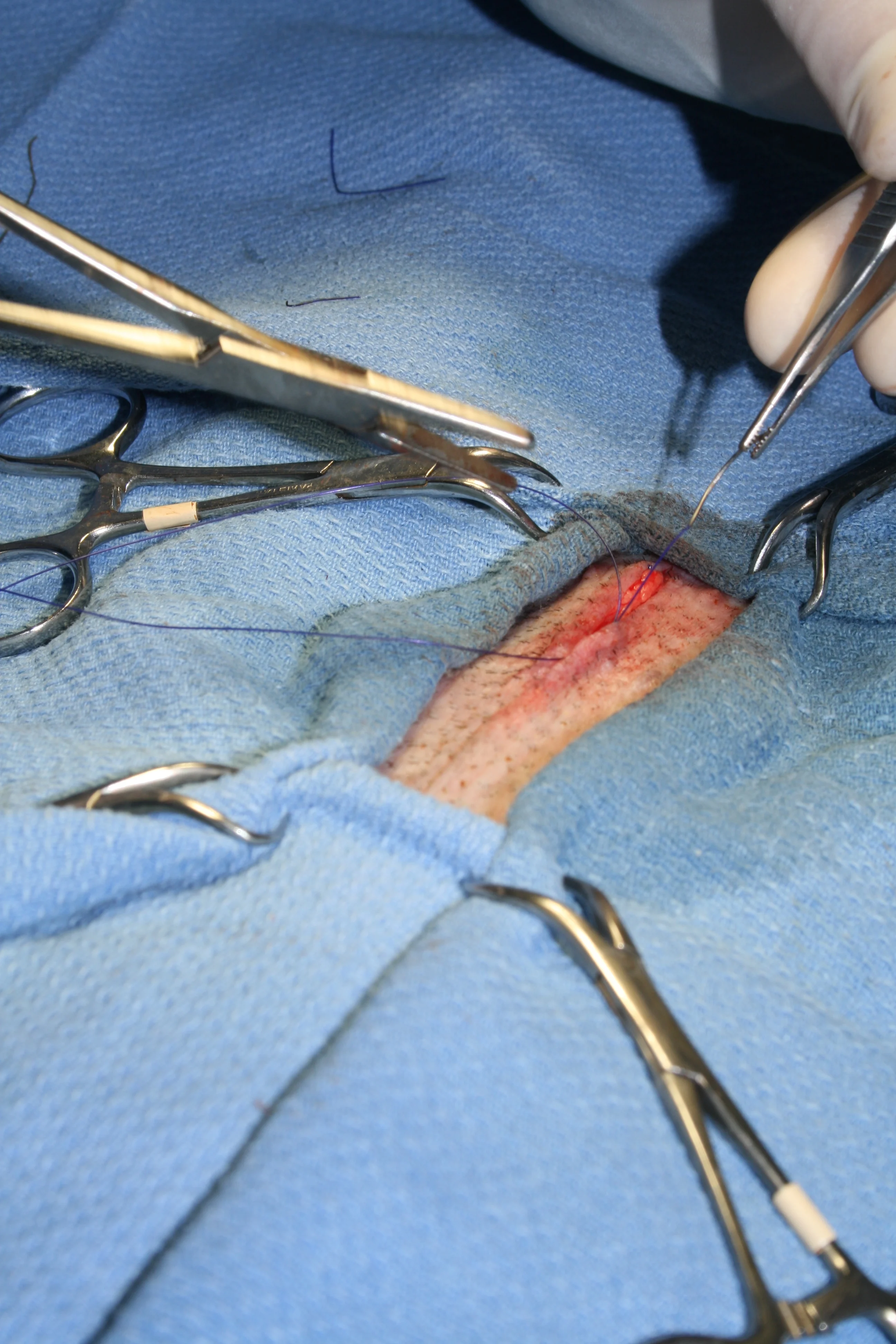
Step 9
Leave patient intubated until swallowing is evident; approximately 10–15 minutes in teacup pigs. Keep patients warm with kennel heaters and blankets. Patient should stay on clean bedding at least 2 weeks postoperatively.
PDS = polydioxanone suture