Chronic Cough in a 3-Year-Old Labrador Retriever
Gary A. Conboy, DVM, PhD, DACVM, Atlantic Veterinary College
Jennifer Caldwell, RVT, RLAT, Kingfisher International
Jonathan Hare, DVM, PhD, Kingfisher International
Donald Martin, PhD, IDEXX Laboratories
Salah Al Izzi, BVMS, MSc, PhD, IDEXX Laboratories

Buddy, a 3-year-old castrated Labrador retriever, was presented for a 2-week history of chronic, intermittent coughing.
History
Buddy, who was presented in February, lived in a rural environment about 50 km (31 miles) north of Toronto, Ontario, Canada, with 1 other dog and 1 cat in the household. Neither of the other animals showed signs of illness. Vaccinations were up-to-date. The dog had previously been on monthly heartworm prevention (ivermectin–pyrantel) from May through October. Buddy had been receiving corticosteroids (prednisone, 1.0 mg/kg PO once a day) to treat atopy for 1 month prior to presentation. His appetite and general demeanor were normal. No exercise intolerance or dyspnea was reported.
Examination
Buddy appeared bright and alert and was moderately overweight. A nonproductive cough could be elicited on tracheal palpation. Temperature, pulse, and respiratory rate were normal; other than the cough, no abnormalities were detected on physical examination.
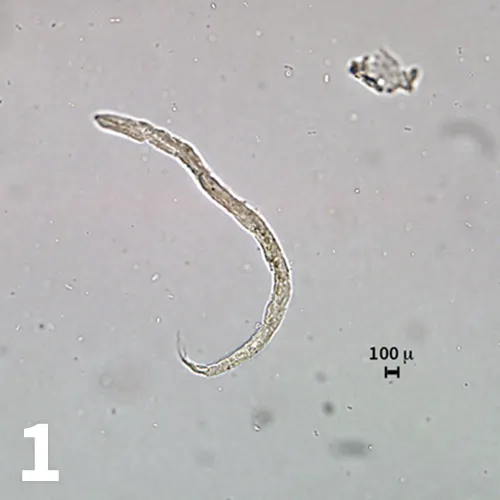
Figure 1. First-stage larva detected on zinc sulfate centrifugal fecal flotation. The osmotic damage from the high specific gravity flotation media is too severe to allow identification (original magnification 20× objective).
Diagnostics
A fresh fecal sample was submitted just before the appointment. A zinc sulfate centrifugal flotation was pursued to detect any possible pulmonary capillarid infection. Eggs of Trichuris vulpis and Alaria spp and several nematode larvae were detected on the centrifugal flotation examination (Figure 1). Identification of the nematode larvae was not possible owing to the effects of the osmotic damage caused by the high specific gravity flotation media.
Figure 2. Dead and degenerating first-stage larva recovered by fecal sedimentation. Larva is about 245 microns in length and has a bluntly rounded anterior end (original magnification 40× objective).
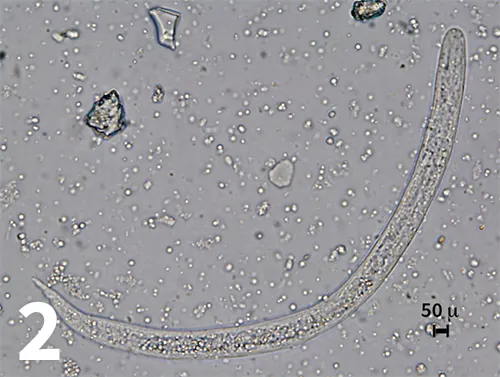
A fecal sedimentation test was then performed, and a Baermann examination was set up in an attempt to obtain undamaged larvae. All larvae recovered on sedimentation were dead and too degenerated to identify. The larvae were 235 to 245 microns in length, and the anterior end was bluntly rounded (Figure 2). The results of the Baermann examination obtained the following day were negative. CBC and serum chemistry profile were within normal limits. Thoracic radiographs revealed a diffuse pulmonary bronchointerstitial pattern.
Figure 3. Live, motile first-stage larva detected in the transtracheal wash sample. Note the bluntly rounded anterior end, a long esophagus (over half the length of the larva), and the slight kink in the tail (original magnification 20× objective).
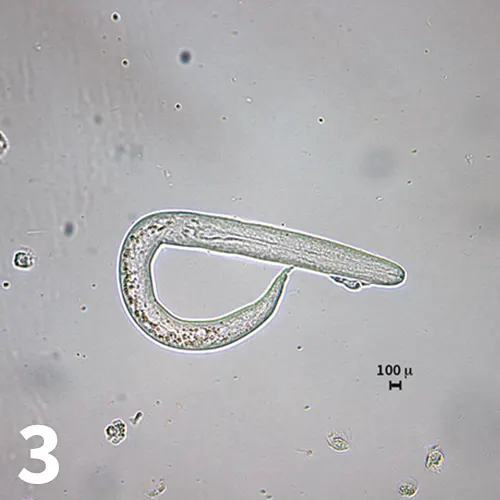
A transtracheal wash sample was obtained. The sample was highly cellular with a predominance of eosinophils. Moderate numbers of neutrophils; small numbers of macrophages, lymphocytes, and plasma cells; and rare mast cells were also present. A moderate number of larvated nematode eggs and larvae were recovered. Gentle pressure on the coverslip induced the release of many of the larvae from the eggs. The larvae were similar in size to those recovered from the fecal sedimentation but were motile, and the morphology could be evaluated in greater detail (Figure 3). The larvae had a bluntly rounded anterior end, long rhabditiform esophagus (over half the total length of the larvae), and a slight kink in the tail. The size and morphology were consistent with first-stage larvae of Filaroides hirthi, Filaroides milksi, and Oslerus osleri (formerly Filaroides osleri).
Related Article: Bronchoalveolar Lavage Fluid Collection Using a Blind Technique
A presumptive diagnosis of Filaroides hirthi infection was made based on clinical signs and history of therapy with an immunosuppressive drug (ie, prednisone).
ASK YOURSELF
In cases of suspected lungworm infection in North America, which fecal examination diagnostic methods should be used to detect the various lungworm species infecting dogs?
Diagnosis
Presumptive F hirthi infection
Related Article: Features of Parasite Ova or Cysts
Treatment and Outcome
Buddy was treated with fenbendazole (50 mg/kg PO once a day for 14 days) and gradually weaned off prednisone therapy. Clinical signs resolved within the 2-week treatment period. Follow-up treatment with milbemycin oxime (0.5 mg/kg PO once monthly for 4 months) was administered to control the unrelated T vulpis infection.
Discussion
F hirthi is a metastrongyloid that infects the bronchioles and lung parenchyma of dogs. This infection is more commonly associated with research laboratory beagle colonies; diagnoses in client-owned animals are relatively rare.1 Infections are usually subclinical; however, life-threatening cases occur if there is immunosuppression from concurrent disease processes (eg, distemper, neoplasia, severe trauma) or therapy (long-term corticosteroid treatment).2-8 Because infection is usually subclinical and diagnosis is difficult, prevalence of infection may be underestimated. Unlike the situation with most other metastrongyloids, the life cycle of F hirthi is direct; dogs become infected through ingestion of first-stage larvae passed in fresh feces or sputum from infected dogs.9 Severity of infection in immunosuppressed dogs is likely related to autoinfection leading to hyperinfection.1
F hirthi is a metastrongyloid that infects the bronchioles and lung parenchyma of dogs.
Failure to detect first-stage larvae by Baermann examination is typical of F hirthi infection in dogs. Detection by the Baermann method requires larvae to be alive and active; larvae of F hirthi and the closely related O osleri and F milksi lack sufficient vigor to exit the feces and be detected by Baermann examination.9 Centrifugal flotation using zinc sulfate is more reliable than the Baermann method for detecting larvae in feces; however, false negatives are common and morphology may be damaged, preventing identification (as in this case).1 Detection of larvae and larvated eggs in sputum or transtracheal wash samples is the diagnostic method of choice. Morphologically, the larvae of F hirthi closely resemble those of O osleri and F milksi.1 Definitive diagnosis of O osleri infection is made by observing wart-like nodules (containing the adult worms) clustered at the bifurcation of the trachea via bronchoscopy.9 Detection of larvae consistent with F hirthi/O osleri and the absence of wart-like nodules at the bifurcation would indicate F hirthi infection.
The client refused consent for bronchoscopy for economic reasons and because of the strong positive clinical response to fenbendazole therapy. F hirthi, the most likely possibility in this case, remains a presumptive diagnosis based on the history of corticosteroid treatment.
Reports of F milksi infection in dogs are rare, and the role it plays (if any) in cases such as this remains unknown. Clinical disease from O osleri infection in dogs usually occurs in animals younger than the dog in this case.10 Other nematode larvae detected in the feces of dogs include Strongyloides stercoralis, Crenosoma vulpis, and Angiostrongylus vasoirum.11 The larvae of S stercoralis and C vulpis have simple straight tails in contrast to the kinked tails of F hirthi.11 The tails of A vasorum are also kinked but differ from F hirthi in that they contain a dorsal spine.11
Fenbendazole (50 mg/kg PO once a day for 14–21 days or 100 mg/kg PO once a day for 7 days) has been used successfully to treat F hirthi infection in dogs.1 Albendazole and ivermectin have also been used to treat dogs infected with F hirthi.1,7
Related Article: Chronic Cough in a Puppy
DID YOU ANSWER?
Baermann examination (Angiostrongylus vasorum L1, Crenosoma vulpis L1), centrifugal flotation (Eucoleus aerophilus eggs, Filaroides hirthi L1, Filaroides milksi L1, Oslerus osleri L1), and fecal sedimentation (Paragonimus kellicotti eggs).
Related Article: Transoral Tracheal & Transtracheal Airway Wash