Whipworm in a Dog: Could It Be More?
Rachel Cooper, DVM, DACVIM, InTown Veterinary Group MetroWest, Natick, Massachusetts

Millie, a 5-year-old, 25-kg spayed Labrador retriever, was presented for a 3-week history of intermittent vomiting and diarrhea.
Being an excellent veterinary diagnostician depends on a solid foundation in the basics. Explore more history-taking challenges in our General Practice Skills series here.
History
Vomiting occurred 3 to 4 times per week and consisted of bile and food. Diarrhea was watery with mucus and minimal blood. The frequency of diarrhea increased to q4–5h with apparent urgency. The diarrhea waxed and waned in severity, with the feces sometimes appearing normal in consistency. Treatment with a diet of boiled chicken and rice was initiated without improvement. No history of dietary indiscretion was noted, and Millie had a normal appetite at home. No other abnormalities were noted. Vaccinations were up-to-date, and the dog received monthly heartworm (ivermectin 0.006 mg/kg PO) and topical flea preventives (imidacloprid 10 mg/kg and pyriproxyfen 0.5 mg/kg). She was kept indoors with free access to the client’s one-acre fenced yard.
Examination Findings
The dog was quiet, alert, and responsive. Heart rate, respiratory rate, and temperature were within normal limits. The dog had tacky mucous membranes and appeared approximately 5% dehydrated. The BCS was 3/9. Although abdominal musculature was tense on palpation, no pain was evident. Rectal examination revealed mucoid diarrhea with minimal frank blood.
Diagnostic Results
CBC results were unremarkable except for mature neutrophilia (See table below). A serum biochemistry profile was within reference range except for moderate hyponatremia and hyperkalemia, and urinalysis revealed no abnormalities. Abdominal radiography showed fluid-filled intestines. An ACTH stimulation test using cosyntropin at 5 µg/kg IV with pre- and postcortisol measured at baseline at 1 hour was within normal limits. Fecal flotation with zinc sulfate centrifugation showed several ova consistent with Trichuris vulpis.
Ask Yourself
What are the differentials for hyponatremia and hyperkalemia?
How are T vulpis and its eggs identified?
What is the life cycle of T vulpis?
What is the treatment for T vulpis?
Diagnosis
Pseudohypoadrenocorticism secondary to T vulpis (whipworm) infection
Treatment
The dog was hospitalized and received 0.9% NaCl at 120 mL/hr to provide maintenance fluids and correct for dehydration and fluid losses. After 24 hours, the dog had not vomited and was released on fenbendazole at 50 mg/kg PO q24h for 3 days, every month for 3 months.
Outcome
At recheck examination 3 days after discharge, the dog appeared clinically normal with resolution of both diarrhea and vomiting. She was still thin but had gained 0.5 kg since the initial examination. Electrolyte abnormalities had completely resolved.
T vulpis ova are extremely resistant and can survive in soil for several years. Dogs kept in contact with contaminated surfaces can be reinfected after treatment. For additional protection in this case, heartworm preventive was switched to milbemycin oxime, which is effective against Trichuris spp infection. The owner was advised to quickly pick up feces to prevent additional contamination and present the dog q6–12mo for fecal examination to rule out recurrence.
Although uncommon in adult dogs, Trichuris spp infection should be considered in cases of acute or chronic GI disease. In dogs with both intestinal signs and a Na:K ratio <27, hypoadrenocorticism or other differentials should be excluded (See Differentials for Low Na:K Ratio below). Even with negative fecal results, dogs should be dewormed because of intermittent parasite shedding, incorrect fecal flotation solution or laboratory technique, watery stool diluting ova, and dated samples.
Differentials for Low Na:K Ratio3
Hypoadrenocorticism
Primary GI disease—including T vulpis infection
Pleural, peritoneal, or pericardial effusion
Renal failure
Heart failure
Pancreatitis
Diabetic ketoacidosis
Uroabdomen
Pyometra
Neoplasia
Late gestation pregnancy
Ask Yourself
1. What are the differentials for hyponatremia and hyperkalemia?
The most common differential for hyponatremia and hyperkalemia with a Na:K ratio of <27 is hypoadrenocorticism. The electrolyte disturbance in this case is most likely from a primary decrease in aldosterone production (although aldosterone was not measured). Primary GI disturbances in dogs with whipworm infection or salmonellosis have been associated with clinical signs and blood work abnormalities similar to those of hypoadrenocorticism, but the mechanism of action for the characteristic electrolyte disturbances is not the same as that for hypoadrenocorticism. Therefore, this differential is called pseudohypoadrenocorticism.
In dogs with GI disturbances, the mechanism of action is thought (although rarely confirmed) to be sodium and bicarbonate-rich fluid loss from the intestines, leading to dehydration. Subsequent thirst leads to dilutional hyponatremia. Hyponatremia and hypovolemia lead to a decrease in potassium excretion, which causes hyperkalemia. Hyperkalemia is also exacerbated by metabolic acidosis, which causes potassium to shift extracellularly in exchange for hydrogen ions.
2. How are T vulpis and its eggs identified?
T vulpis eggs (See Figure 1) are most commonly identified on fecal flotation. Direct saline smear examination is insensitive, with one study showing 92.7% false-negative results when comparing direct smears to fecal centrifugation.1 T vulpis eggs are barrel or football shaped and yellow-brown with prominent bipolar plugs. Trichuris ova are smooth shelled and measure ~79 x 38 µm.
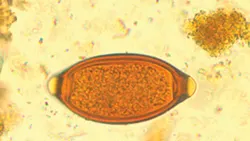
T vulpis eggs are yellow–brown and barrel or football shaped with prominent bipolar plugs.
In humans T vulpis has been identified on ultrasonography, and a recent case of a dog with T vulpis described intestinal loops distended with fluid and several thin linear hyperechoic echoes floating within the bowel loop.1 Ultrasonography should not be considered diagnostic for T vulpis infection, but similar findings may increase clinical suspicion.
3. What is the life cycle of T vulpis?
T vulpis has a direct life cycle. Embryonated eggs are ingested from a contaminated environment and hatch into larvae in the small intestine, where they attach to the mucosa and mature for 2 to 10 days. They then reenter the lumen and migrate to the cecum, terminal small intestine, or colon to mature. The prepatent period is 74–90 days. Eggs are unembryonated when they are initially passed in feces; they embryonate 9–21 days after being passed and are then infectious. Adult female whipworms (See Figure 2) can produce up to 2,000 eggs per day.2

Adult whipworm
4. What is the treatment for T vulpis?
Treatment of T vulpis is provided either with praziquantel–pyrantel pamoate–febantel q24h or fenbendazole q24h for 3 days. Because young larvae may be more resistant than adults to anthelmintics, deworming should be performed monthly for 3 months. This is longer than recommended for other parasites because of the prolonged prepatent period.