Regenerative Therapy for Canine Myocardial Disease
Regenerative medicine involves the use of stem cell and gene-replacement therapy—either as single modalities or in combination—to manipulate the body’s capacity for repair. Stem cells are undifferentiated, self-renewing cells that possess a multi-lineage differentiation potential with the capacity to regenerate, repair, or substitute damaged tissue, allowing the re-establishment of its function.
Related Article: Stem Cells: New Therapy for Old Diseases
The mechanism of action of stem cells involves several pathways1 and is largely dependent on the type of cell (eg, skeletal myoblasts, bone-marrow–derived cells, embryonic stem cells, endogenous cardiac stem cells, and induced pluripotent stem cells) (Figure 1).

Figure 1. Types of stem cells used for cardiac regenerative therapy to restore heart function directly. Multipotent and unipotent stem cells originating from skeletal myoblasts (A), bone marrow and blood endothelial progenitor cells (B), adipose-derived cells (C), resident cardiac stem cells (D), and bone marrow (E). Images courtesy of Paola Longo, MD, head of the vascular diagnostic operative unit at San Filippo Neri Hospital, Rome, Italy.
For example, pluripotent stem cells are capable of differentiating into all cell types, including cardiomyocytes, but multipotent and unipotent stem cells can only differentiate into a limited number of cell types. Cardiac stem cells are capable of differentiating into myocytes, endothelial cells, and vascular smooth muscle cells.
Gene-replacement therapy has the potential to become an ideal treatment for inherited diseases for which the mutant gene has been identified. It has traditionally been used to transfer a gene that encodes a functional protein into a diseased patient to produce long-term expression of the deficient protein2,3 using a viral vector (eg, adeno-associated virus [AAV]); this can regenerate lost tissue (Figure 2).

Figure 2. Therapeutic gene transfer using viral vectors for gene delivery reengineers the virus to replace the viral disease-causing genes with the gene of interest (ie, the therapeutic gene). In this simplified schematic, an AAV (A) has its viral genes removed (B) and a therapeutic gene of interest added (C). This viral vector package containing therapeutic genes is injected into a patient (D). The vector virus binds to host cells and introduces therapeutic genetic material (and directions for producing more copies of the virus) as part of the replication process (E).
Indications and Advantages
In cardiovascular medicine, most cardiac stem cell therapies have been directed toward myocardial repair following acute and chronic myocardial infarction in humans. Studies in cardiac stem cell therapy have shown that transplantation of mesenchymal stem cells improves cardiac function in rats, rabbits, and humans with dilated cardiomyopathy.4-9
Dilated cardiomyopathy (DCM) is the most common adult-onset acquired myocardial disease that affects large- and giant-breed dogs. Doberman pinschers are affected by the most common and severe form of DCM in veterinary medicine.10 The disease can progress to cause refractory congestive heart failure or sudden death.
Conventional palliative medical therapy with diuretics, ACE-inhibitors, inodilators, and anti-arrhythmic drugs has assisted in management of these cases but does not correct underlying cardiac muscle cell dysfunction. Advanced therapeutic strategies used in human medicine, such as cardiac transplantation, implantation of mechanical-assist devices, and cardiac resynchronization therapy, are invasive and generally cost prohibitive for veterinary patients.
Related Article: Stem Cell Therapies: Reality in the Making
A specific genetic mutation (pyruvate dehydrogenase kinase gene [PDK4]) has been identified in Doberman pinschers with DCM.11 This offers a potential new avenue for research aimed at identifying better treatment options for this disease.
Regenerative therapy with gene-replacement therapy alone or in combination with stem cells has been used with various levels of success to treat diseases where a specific genetic mutation has been identified (eg, canine hemophilia, lysosomal storage diseases, inherited retinal diseases12-18). This may also be a viable treatment option for Doberman pinschers with DCM and the recently identified PDK4 mutation as well as other cardiac diseases in veterinary medicine for which a genetic mutation has been identified.
Challenges and Disadvantages
Challenges of global cardiac regeneration using stem cells and gene therapy involve 4 areas:
Identification of ideal cell types and vectors
Identification of the dose required for global cardiac regeneration
Identification of the best route of cell and/or vector delivery
Development of a safe and effective product.
A rational approach to the study of cardiac disease is needed to understand the mechanisms that may improve myocardial performance. A wide variety of cell types (eg, skeletal myoblasts, bone-marrow–derived cells, embryonic stem cells, endogenous cardiac stem cells) and vectors (eg, AAV2, AAV6, AAV8, AAV9) have been considered as candidates for therapeutic delivery. It is still unknown which vector, type of stem cell or progenitor cell, or some combination of the 2 is the best option for achieving cardiac regeneration.
Cell numbers required to regenerate the entire myocardium and how many cells can be safely used is still unclear with regard to cell therapy in the treatment of global cardiac dysfunction.
Cardiomyocyte transduction (ie, the introduction of DNA into the cell via a viral vector) has proven more difficult in global cardiac dysfunction because myocardial volume is a determinant of the proportion of the myocardial mass that is transduced by the administration of a cell and/or vector. The percentage of the myocardium required to be successfully transduced for effective therapy is still unclear, and the required number of transduced cells may vary depending on the underlying cause of cardiomyopathy.19
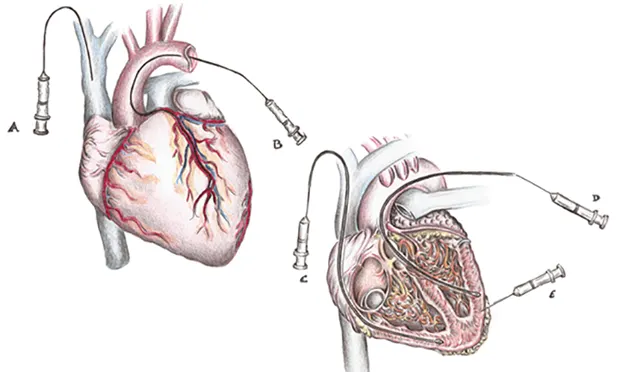
Finding the appropriate dose is not the only determinant of good outcome. Another challenge for cardiac regeneration is determining the optimal route of delivery. Retention of cells immediately after delivery is highly dependent on the delivery strategy. Cells can be injected intravenously, into coronary arteries, or directly into the myocardium (Figure 3).

Figure 3. Retention of cells immediately after delivery is highly dependent on the delivery strategy. Cells can be injected through several methods. Intravenously (A) is simplest and least invasive, but dilution of cells and/or vector by systemic blood circulation and cells and/or vectors uptake by other organs can pose potential challenges. Coronary artery infusion (B), in which the cells and/or vector are injected through the lumen of an inflated angioplasty catheter into the coronary artery and reside in the coronary circulation until balloon is deflated. Retrograde coronary venous sinus infusion (C), in which the cells and/or vector are injected into the coronary sinus through the lumen of an inflated angioplasty catheter under pressure to disrupt endothelial borders and allow cells and/or vector to traverse into the myocardium. Direct intramyocardial involves injection of the cells and/or vector either endocardially (D) or epicardially (E). The primary advantage of this method is that cells and/or vectors delivery bypasses the endothelial barrier, which results in high local concentrations at the injection site.
The IV route is safest and easiest, but studies have shown minimal dwell time, non-specific cardiac muscle targeting, and poor results. A large number of cells and/or vectors is required to achieve cardiac transduction to counter first-pass effect; this exponentially increases the cost of the treatment. There is also a concern regarding the intracoronary delivery route; administration in this manner may result in blockage of the coronary arteries and cause further damage to the myocardium.20,21
The development of an effective and safe product (a combination of a vector and stem cell or gene) is of utmost importance. The increased popularity of AAV vectors has prompted the FDA to regulate procedures, practices, and facilities for the implementation of methods that avoid genetic exchange between animals and humans.
Clinical Impact
Regenerative therapy for cardiac disease is a growing area of research that has recently led to several clinical trials in humans. Strategies such as cell transplantation and reprogramming have demonstrated intriguing and exciting results.
When a specific genetic mutation is discovered, the use of a combined approach of stem cell and gene-replacement therapy to achieve cardiac regeneration is a real possibility that could change the progression of the disease.
Future Directions
Not long ago, the heart was still considered a static and postmitotic organ that lacked regenerative capacity; it was thought that the number of cardiomyocytes in an individual was established at birth, and cardiomyocyte hypertrophy was considered the only cellular adaptive response of the heart.22
After more than a decade of research, views have changed comprehensively regarding cardiac remodeling and cardiac regenerative therapy as an important area of research. Several experimental studies were initiated with different types of stem cells. Because of the encouraging results, a rapid transition from preclinical experiments to clinical trials occurred.23 Although clinical trials demonstrated that cardiac regenerative therapy is safe, it remains unclear why preclinical expectations were not met in the majority of the studies.23
The results of clinical trials and experiments demonstrate that much remains to be investigated before clinical applicability can become a reality.
AAV = adeno-associated virus, ACE = angiotensin-converting enzyme, DCM = dilated cardiomyopathy, PDK4 = pyruvate dehydrogenase kinase gene
LUIZ BOLFER, DVM, is cardiology resident and PhD student at University of Florida, where he studies veterinary clinical sciences with an emphasis in cardiac gene therapy. His research interests include extra-corporeal blood purification therapy, myocardium dysfunction and failure, and cardiac regenerative medicine. A graduate of Universidade Tuiuti do Paraná, Dr. Bolfer completed a small animal medicine and surgery internship at University of Illinois and a residency in emergency and critical care at University of Florida.
AMARA ESTRADA, DVM, DACVIM (Cardiology), is associate professor and service chief of the cardiology department in the small animal hospital at University of Florida. Dr. Estrada’s research interests include electrophysiology, pacing therapy, complex arrhythmias, cardiac interventional therapy, and cardiac regenerative medicine. Dr. Estrada graduated from University of Florida and completed a cardiology residency at Cornell University.