Pimobendan & Degenerative Valve Disease: An EPIC Update
Ashley E. Jones, DVM, DACVIM (Cardiology), Veterinary Specialty Center, Buffalo Grove, Illinois
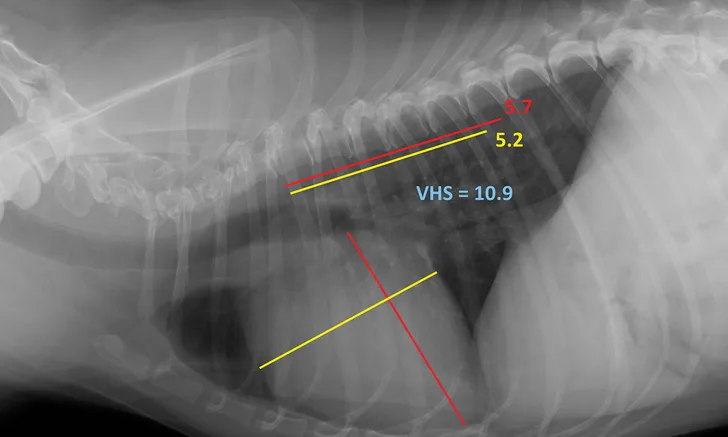
Measurement of VHS on a patient diagnosed with degenerative valve disease (via echocardiogram) that would meet the criteria for radiographic evidence of cardiomegaly according to the EPIC study criteria (ie, VHS >10.5)
In the Literature
Boswood A, Häggström J, Gordon SG, et al. Effect of pimobendan in dogs with preclinical myxomatous mitral valve disease and cardiomegaly: the EPIC study—a randomized clinical trial. J Vet Intern Med. 2016;30(6):1765-1779.
The Research …
Myxomatous mitral valve disease (MMVD), a common heart disease in dogs, is characterized by a long preclinical phase, and no therapies have been definitively proven to prolong this time before onset of congestive heart failure (CHF).
Figure 2 Measurement of the left atrial:aortic root ratio on a patient diagnosed with degenerative valve disease that would meet the criteria for left atrial enlargement according to the EPIC study criteria (ie, LA/Ao >1.6)

Dogs (n = 360) were enrolled in this study (Evaluation of Pimobendan In dogs with Cardiomegaly caused by preclinical mitral valve disease [EPIC]) to compare the effectiveness of pimobendan vs placebo in delaying onset of CHF signs, cardiac-related death, or euthanasia. Dogs (9.1-33.1 lb [4.1-15 kg] body weight) were admitted if they were diagnosed with MMVD based on the presence of a grade 3/6 or greater left apical systolic murmur, echocardiographic evidence of degenerative changes to the mitral valve apparatus, and presence of mitral regurgitation. They also had to have sufficient cardiomegaly meeting specific echocardiographic and radiographic parameters for left atrial and left ventricular dilatation (Figures 1 and 2).
Dogs were administered pimobendan or placebo at 0.4-0.6 mg/kg PO, divided and given at 12-hour intervals. Each patient was determined to have reached the end of the study (ie, primary endpoint) if diagnosed with CHF via thoracic radiography or if they died or were euthanized due to cardiac disease.
An interim analysis demonstrated a clear treatment benefit for patients in the pimobendan group, so the study was terminated early. The pimobendan group had a median time to primary endpoint of 1228 days as compared with 766 days for the placebo group. There also was no difference in adverse events, indicating pimobendan was well tolerated with minimal side effects (the most common being vomiting and diarrhea).
This study demonstrated the benefit of pimobendan in significantly prolonging the time to onset of CHF or cardiac-related death in dogs with cardiomegaly due to MMVD.
… The Takeaways
Key pearls to put into practice:
In patients with suspected MMVD based on the presence of a left apical systolic murmur in a middle-aged to older dog, thoracic radiographs are a beneficial first line to assess for cardiomegaly using vertebral heart size (VHS) measurement.
Echocardiography is essential to confirm diagnosis of MMVD and give detailed assessment of specific left atrial and left ventricular chamber enlargement criteria.
Chronic oral therapy with pimobendan should be initiated at 0.4-0.6 mg/kg (divided into q12h dosing) in patients without clinical signs that are diagnosed with cardiomegaly due to MMVD.