Clinical Skills
Discover guidance for assessing patients, diagnostic testing, surgical procedures through expert-led articles and videos. Perfect for general practitioners, medical students, and residents looking to refine their skills.

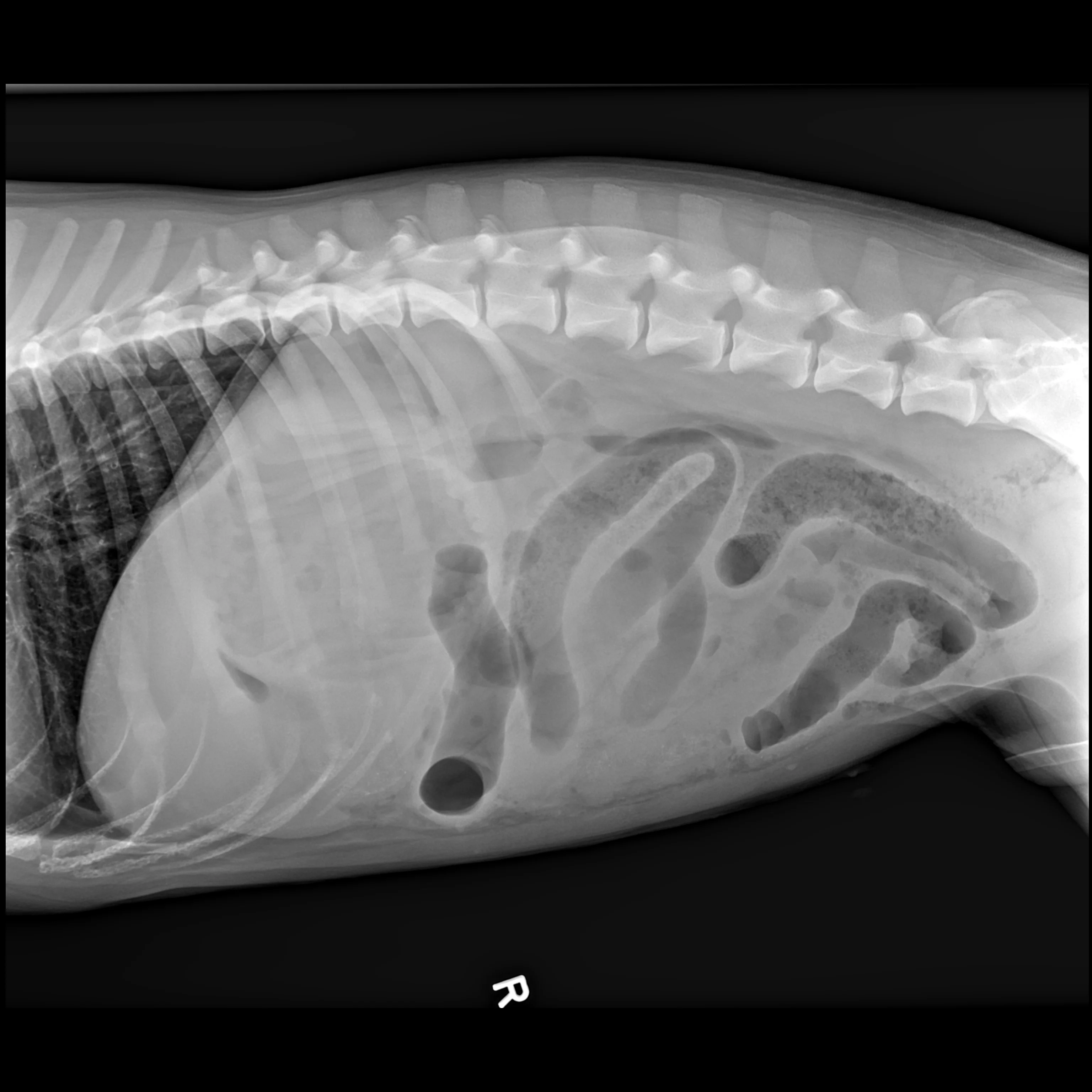
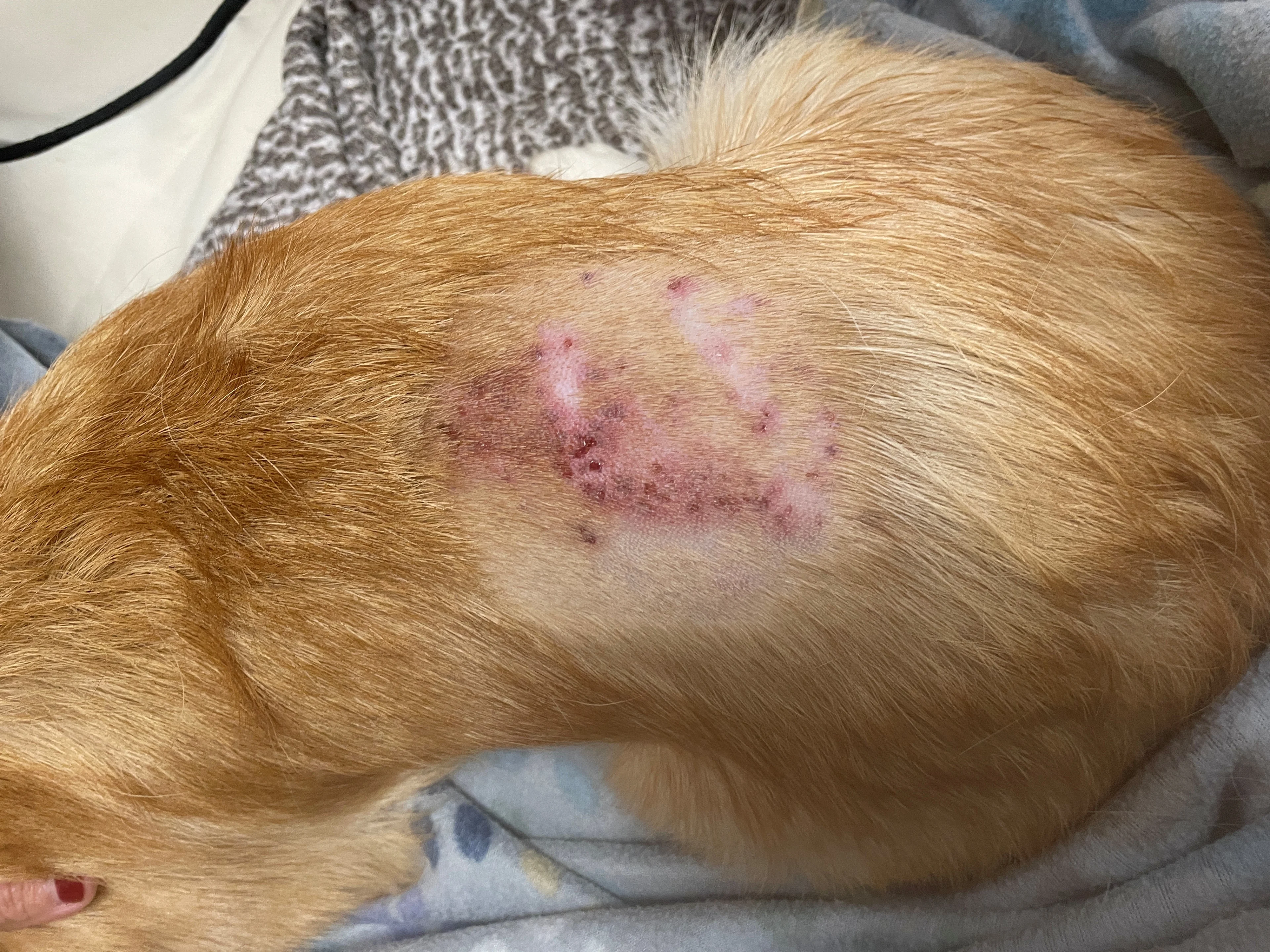
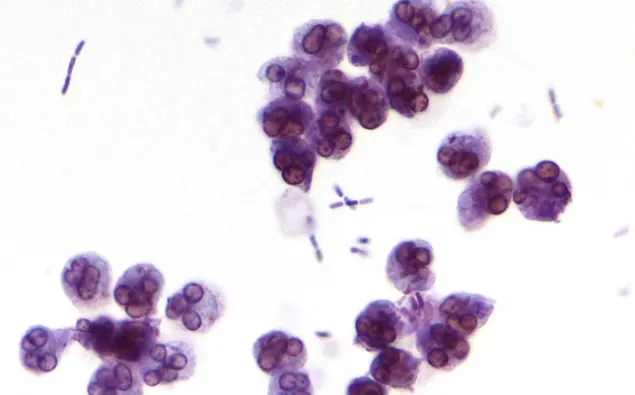
Dr. Seitz shares excellent advice on how to use radiography to the fullest—even if it means getting a wooden spoon or carbonated beverage—and how to apply point-of-care ultrasound at the general practice level.
Topics In Clinical Skills
Featured in Clinical Skills
New in Clinical Skills
Use Plumb’s™ to take your practice further
From the team that brings you Clinican’s Brief, extend your knowledge with everything from diagnostic and treatment guidance to reliable drug information and pet owner education. Plumb's™ is the easy-to-use tool you can rely on to find the best path forward for every patient.
Drug Monographs
Drug Interaction Checker
Medication Guides
Clinical Briefs
Algorithms
Patient Guides