Triaditis Syndrome
Feline triaditis is the syndrome of concurrent cholangitis, pancreatitis, and inflammatory bowel disease (IBD).
The prevalence of triaditis in necropsied cats is relatively high,1,2 although these findings should be interpreted with caution in light of (1) potential selection bias in cats with neutrophilic cholangitis or suppurative pancreatitis and because (2) histopathologic findings of pancreatitis may not always correlate with clinical disease. The temporal nature of the relationship as well as the specific cause(s) of the IBD, cholangitis, and pancreatitis have not been well-elucidated to date.
The unique anatomy of the feline common bile duct and pancreatic duct joining as a common single duct before entering the duodenal papilla increases the risk for retrograde infection in the liver and pancreas.
Pancreatitis is usually sterile, although the role of bacteria in the pathogenesis of pancreatitis remains unknown.2,3 Recent studies suggest that ascending passage of bacteria or bacterial products from the inflamed intestine may play a role in the development of pancreatitis,3-5 and it is likely that the retrograde ejection of bile into the pancreatic and common bile ducts during vomiting increases the risk for pancreatic inflammation and cholangitis. The unique anatomy of the feline common bile duct and pancreatic duct joining as a common single duct before entering the duodenal papilla increases the risk for retrograde infection in the liver and pancreas.<sup4 sup>
Related Article: Canine Pancreatitis
Histopathologic studies have described pancreatitis in 50% of cats with cholangitis and in 39% of cats with IBD.6,7 In addition, up to 83% of cats with cholangitis have concurrent IBD.7 Cats with triaditis are at increased risk for developing secondary hepatic lipidosis.8 A study of 29 cats (8 healthy and 21 clinically ill animals) confirmed pancreatitis in 18 cats, of which 19% had concurrent lipidosis, 63% had intestinal inflammation, and 75% had hepatic inflammation, suggesting a high prevalence of concurrent disease.<sup1,8 sup>
Clinicians must consider underlying pancreatitis and concurrent IBD in all cats with hepatic lipidosis or cholangitis even if liver disease is the primary disorder being investigated. Clinical signs in all 3 conditions are vague with tremendous overlap, underscoring the importance of performing a comprehensive workup to rule out each of these disorders in affected cats.
Feline Cholangitis
Inflammation centered on the biliary tree is a common form of hepatic disease and appears to be the second most common form of liver disease (after hepatic lipidosis) in cats in North America.9 A classification scheme proposed by the WSAVA Liver Diseases and Pathology Standardization Research Group recognizes 3 distinct forms of cholangitis in cats: neutrophilic cholangitis, lymphocytic cholangitis, and chronic cholangitis associated with infection by liver flukes.<sup10 sup>
Neutrophilic cholangitis is subcategorized into acute vs chronic types based on histopathologic evaluation: Acute represents a primarily neutrophilic infiltrate, and chronic represents a mixed inflammatory infiltrate consisting of increased plasma cells, lymphocytes, and macrophages.
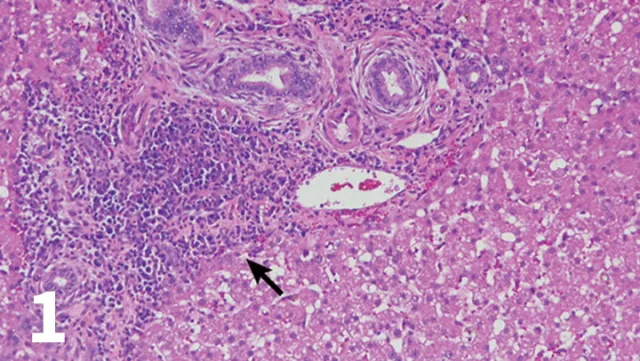
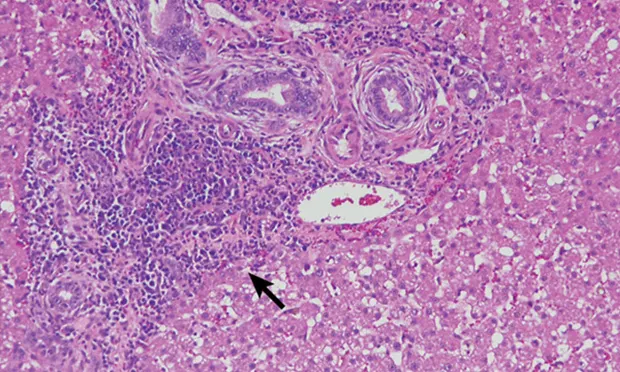
H&E-stained section (20× objective ) of a feline liver with periductal lymphocytic inflammation (arrow) centered around portal areas with concentric rings of fibrosis consistent with lymphocytic cholangitis.
The chronicity of lymphocytic cholangitis is believed to represent a later stage of neutrophilic cholangitis or may represent a separate disease entity. It is characterized by a moderate-to-marked infiltration of the portal areas by small lymphocytes with or without biliary hyperplasia, portal or periductal fibrosis, or bridging fibrosis (Figure 1). The underlying cause is unknown, but 1 possible cause is an immune disorder.<sup11 sup>
Related Article: Top 5 Liver Conditions in Cats
Feline Pancreatitis
Pancreatitis has a variety of possible triggers, and a definitive cause has not been identified. Pancreatitis can be acute necrotizing or chronic in nature, with fibrosis and recurrent inflammation. Acute necrotizing and chronic pancreatitis cannot be differentiated clinically or biochemically in cats, and ultrasonographic differentiation can also be challenging.12 In addition, pancreatitis was suspected antemortem in only 30% of 63 cats diagnosed with pancreatitis on histopathologic evaluation,12 which is consistent with the subtle clinical presentation.
Although most cats diagnosed with triaditis tend to have chronic pancreatitis, acute and chronic histopathologic features of pancreatitis can be found in the same animal. A necropsy study evaluating the prevalence and histopathologic characteristics of pancreatitis in 115 cats confirmed acute pancreatitis in 16% of the cats,2 whereas another study documented a much higher prevalence (44%) of concurrent acute and chronic pancreatitis.<sup1 sup>
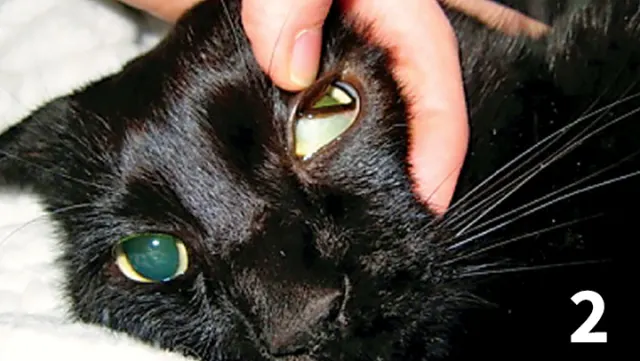
Icteric nictitating membrane in a cat with triaditis.
Feline Inflammatory Bowel Disease
IBD is the most common cause of chronic vomiting and diarrhea in cats. It refers to a group of chronic enteropathies characterized by infiltration of the lamina propria of the GI tract with inflammatory cells (most commonly lymphocytes and plasma cells). The pathogenesis involves a complex interaction among the intestinal microbiota, immune dysregulation, genetics, and environmental triggers.4,12-14
|
Anorexia, lethargy, and weight loss ± vomiting and diarrhea
± Abdominal pain or discomfort
Dehydration
Cholangitis
Fever
Icterus (Figure 2)
Abdominal effusion
Hepatomegaly (although liver is often normal in size)
Pancreatitis
Icterus
Occasional fever
IBD
Thickened bowel loops
Anorexia or polyphagia
|
Diagnostics
Accurate diagnosis of triaditis is based on a comprehensive history, thorough physical examination, and interpretation of laboratory and imaging findings (see Signs and Examination Findings and Laboratory Findings). Intestinal and hepatic biopsies are necessary for histopathologic confirmation of IBD and cholangitis. Intestinal biopsies are also warranted to rule out lymphoma.
Related Article: A Tail of Triaditis
Biopsy specimens may require additional diagnostic testing such as fluorescence in situ hybridization, which can be used to confirm a bacterial infection associated with neutrophilic cholangitis in some cats when bile or liver tissue cultures have been negative.11,13 Immunohistochemistry and clonality can be performed on formalin-fixed biopsies to help differentiate IBD from intestinal lymphoma.<sup15 sup>
Treatment Challenges
Management of triaditis can be challenging in light of the concurrent involvement of the liver, pancreas, and intestinal tract, and therapy should be predicated on the severity and nature of the underlying disorders. Most of these cats have been anorectic for days to weeks, and nutritional support is of paramount importance. Nutraceuticals should be administered to provide S-adenosyl- L-methionine (SAMe) and silybin, for their antioxidant properties. SAMe increases glutathione concentrations, and silybin inhibits formation of superoxide anion radicals and nitric oxide and abolishes the decrease of glutathione, superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase. Silybin has also been shown to be an iron chelator. Oral administration of SAMe to healthy cats leads to an increase in plasma SAMe and liver glutathione.16 Cats with liver disease have been shown to have low glutathione levels.17 No controlled clinical trials, however, have documented efficacy, prolongation of survival, or improved quality of life in cats with cholangitis following administration of nutraceuticals.
Most patients are managed supportively with parenteral fluids (if dehydrated), an enteral feeding device, cyanocobalamin supplementation (if hypocobalaminemic), and antimicrobial therapy pending biopsy results.
How I Diagnose Triaditis Syndrome
STEP 1
Conduct diagnostic testing in suspected cases
CBC, serum chemistry panel, urinalysis, total thyroxine, serum cobalamin (vitamin B12), Spec fPL, FeLV/FIV serology, and abdominal ultrasound
Additional testing based on signs and examination findings
STEP 2
Assess for cholangitis
Abdominal ultrasound to assess liver size and echogenicity, dilation of the gallbladder and/or common bile duct, thickening of the biliary wall, biliary sludge or debris, and enlarged or mottled regional lymph nodes
Abdominal ultrasound can be unremarkable, and this does not rule out disease18
Fine-needle aspiration (FNA) of the liver to screen for neoplasia (lymphoma, mast cell disease, carcinoma) before considering surgical biopsy
Normal FNA does not conclusively rule out neoplasia
Coagulation panel (PT/PTT) before biopsy
Biopsy of multiple liver lobes (via laparotomy or laparoscopy) for histopathology; ultrasound-guided needle biopsy is inferior to biopsy of multiple liver lobes via open surgery or laparoscopy
Bile culture and tissue culture
Collect tissue for histopathology, and submit both tissue and bile samples for aerobic and anaerobic culture and susceptibility
Bile culture is superior to hepatic parenchymal culture
STEP 3
Assess for pancreatitis
Abdominal ultrasound (Figure 3) may show ≥3 of the following features: evidence of pancreatomegaly, hypoechoic parenchyma, hyperechoic surrounding mesentery, irregular pancreatic border, dilation of pancreatic or bile ducts, dilation of the cystic duct, abdominal effusion
A normal-appearing pancreas does not rule out pancreatitis
Repeat abdominal ultrasound should be considered in light of pancreatitis being a dynamic disease process
Abdominal radiographs are insensitive for pancreatitis
Spec fPL is the most sensitive diagnostic test for pancreatitis; however, it is less sensitive with chronic disease1
A normal Spec fPL does not conclusively rule out pancreatitis
Evaluation of Spec fPL in 182 cats in a prospective study found a sensitivity and specificity of about 80% using a cutoff of 5.4 µg/L, supporting its use as a screening test for feline pancreatitis<sup19 sup>
Pancreatic biopsy is considered the gold standard for diagnosis, but it may not be necessary with ultrasound and Spec fPL testing
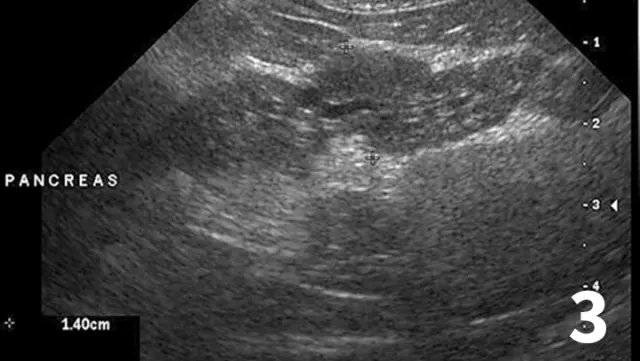
Ultrasound highlighting several features of pancreatitis, including an enlarged pancreas with a hypoechoic parenchyma and hyperechoic surrounding mesentery, as well as a dilated pancreatic duct (arrow). These features are believed to represent acute pancreatitis.
STEP 4
Assess for inflammatory bowel disease
Abdominal ultrasound with careful evaluation of intestinal wall layering:
Specific changes include thickening of the muscularis propria layer with or without concurrent thickening of the submucosal layer (Figure 4)
Determine whether normal intestinal architecture is preserved because loss of layering is more consistent with intestinal lymphoma (large cell)20,21
Evaluate regional lymph nodes carefully for size and echogenicity. Large hypoechoic lymph nodes are supportive of lymphoma
Fecal centrifugation flotation to evaluate for intestinal parasites
Serum cobalamin measurement
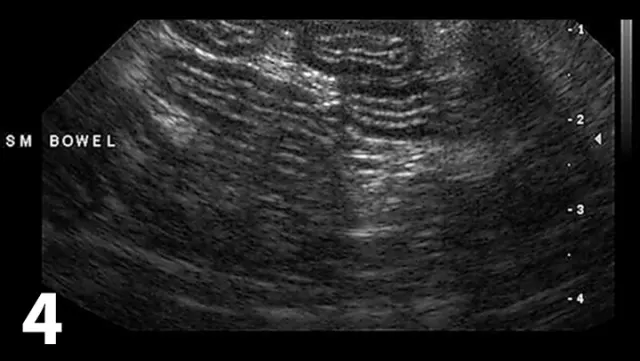
Ultrasound of the small intestine showing concurrent thickening of the muscularis propria layer and submucosal layer commonly seen in patients with IBD and small cell lymphoma.
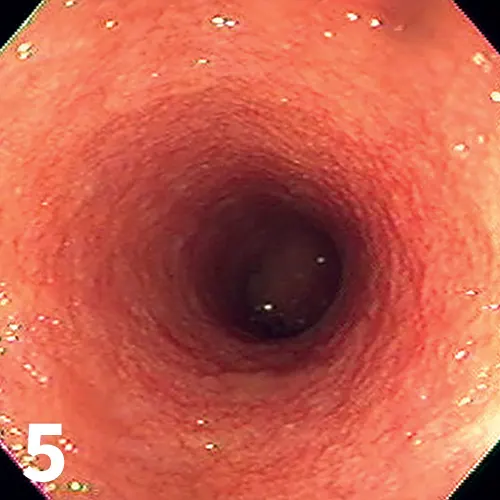
Endoscopic image from a cat with IBD; note the irregular and granular appearance of the duodenal mucosa.
Endoscopic (Figure 5) or full-thickness biopsy of the GI tract for histopathology may be performed after a therapeutic trial of a limited antigen or hydrolyzed diet for 2 to 3 weeks in milder cases
Biopsies are often performed in more severe cases or when clinical signs progress despite dietary management (Figure 6)
Ileal biopsies are warranted in cats that are hypocobalaminemic
Ultrasonographic evidence of abnormal intestinal layering affecting the jejunum should prompt consideration for full-thickness biopsies via laparotomy or laparoscopy
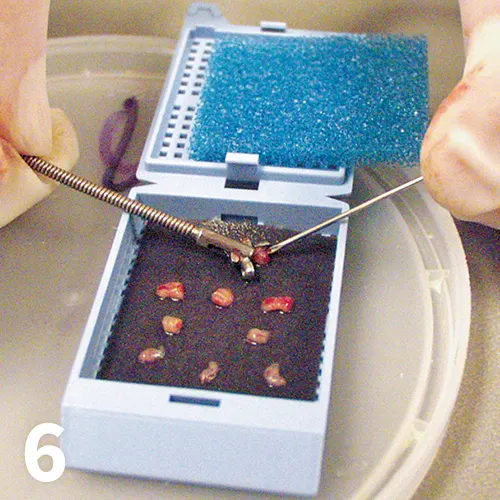
Biopsy cassette demonstrating multiple appropriately sized endoscopically acquired biopsies
How I Treat Triaditis Syndrome
STEP 1
Treat cholangitis
Optimal treatments are largely unknown; recommendations are based on anecdotal evidence and clinical experience
If coagulation abnormalities are present, administer vitamin K1 at 0.5 to 1.5 mg SC twice a day before biopsy for 36 hours or 3 doses
Administer ursodeoxycholic acid 10 to 15 mg/kg PO once a day; this choleretic prevents mitochondrial damage and has antifibrotic and immune-modulating properties
Administer nutraceuticals (SAMe and silybin)
Provide nutritional support
Assisted feeding (forced oral feeding is discouraged for risk of conditioned food aversion)
Appetite stimulants may be used initially to encourage food intake in hyporexic cats, but cats taking appetite stimulants rarely meet their caloric requirements
Mirtazapine, an antiemetic and appetite stimulant that is a serotonergic antidepressant, is dosed at 3.75 mg (a quarter of a 15-mg tablet) PO every 2 to 3 days; side effects include increased activity, behavior changes, vocalization, and tremors
Cyproheptadine, a phenothiazine with antiserotonin properties, is dosed at 2 mg PO once or twice a day; side effects (eg, hyperactivity) are rare
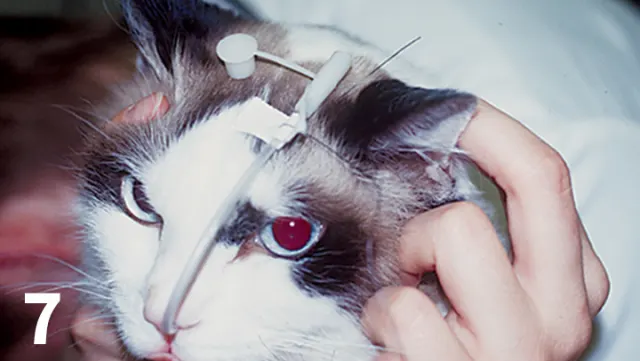
Nasoesophageal feeding tubes are used to provide short-term nutritional support in cats with triaditis.
If anorexia or hyporexia persists for >3 days, consider additional nutritional support
Nasoesophageal or esophageal feeding tubes should be pursued (Figure 7)
Nasoesophageal tubes are best for short-term (<10 days) nutritional support; the tube diameter (5–8 Fr) necessitates liquid enteral formulas only
Diet selection depends on concurrent involvement of bowel (IBD) in which a limited antigen diet is fed
There is debate regarding fat restriction for cats with pancreatitis; this is in contrast to pancreatitis in dogs, in which moderate-to-marked fat restriction is recommended. The authors advocate minimal-to-no fat restriction in cats with pancreatitis
Parenteral nutrition should be considered in severe cases associated with intractable vomiting or severe malassimilation
Manage pain
Buprenorphine 0.01 mg/kg sublingually or SC 2 to 4 times a day
Other opioids may also be considered
The transdermal fentanyl patch (12 µg/hr for small cats and 25 µg/hr for large cats every 72 to 118 hours) for longer-duration pain control
Antiemetics
Maropitant (neurokinin-1 receptor antagonist) at 1 mg/kg SC once a day for cats >16 weeks of age
Ondansetron or dolasetron (5-HT3 receptor antagonists) at 0.5 to 1 mg/kg PO or IV once or twice a day
Antimicrobial therapy is important for acute neutrophilic cholangitis
Treatment is ideally based on culture and susceptibility results and includes amoxicillin–clavulanic acid or amoxicillin and fluoroquinolones for 4 to 6 weeks14
Corticosteroids
Prednisolone at 0.5 to 1 mg/kg PO twice a day initially, tapered slowly over 8 to 10 weeks
In the author’s experience, more aggressive immune modulation may be necessary based on response to corticosteroids alone. Anecdotal reports are available for use of chlorambucil in conjunction with prednisolone in severe cases of lymphocytic cholangitis
If patient is encephalopathic:
Lactulose at 0.5 to 1 mL/kg PO 2 to 3 times a day
Antibiotics (amoxicillin or ampicillin [20 mg/kg PO or IV twice a day])
Protein-restricted diet
If gastric ulceration is suspected, a potent acid suppressant such as omeprazole (1–1.5 mg/kg PO or IV twice a day) should be administered<sup22 sup>
STEP 2
Treat pancreatitis
IV fluids/electrolytes, including isotonic crystalloids to correct dehydration and maintain hydration
Colloids (eg, hetastarch or hydroxyethyl starch) are rarely needed except in severe cases
Analgesics (see above)
Antiemetics (see above)
GI protectants: famotidine (H2-receptor antagonist) 0.5 to 1 mg/kg PO, SC, or IV twice a day or omeprazole (proton pump inhibitor) 1 to 1.5 mg/kg PO or IV twice a day22
Nutritional support (see above)
Prednisolone: 0.5 mg/kg PO once a day to reduce inflammation; may be used at higher doses for longer duration when concurrent IBD or lymphocytic cholangitis is present
STEP 3
Treat IBD
Nutritional support (see above)
Dietary therapy using a limited-antigen intact protein or hydrolyzed diet
Antimicrobial therapy
Metronidazole at 10 mg/kg PO twice a day
Tylosin at 10 mg/kg PO once a day
Vitamin B12 at 250 µg SC once a week for 6 weeks, followed by 250 µg every 3 weeks if warranted (based on repeating serum cobalamin testing immediately before 3-week dosing is administered)
Corticosteroids often pursued if appropriate therapeutic trial with diet and antimicrobials has failed or with severe disease
Prednisolone is dosed at 0.5 to 1 mg/kg PO twice a day initially and tapered over 8 to 10 weeks
More aggressive immune modulation may be necessary based on response to corticosteroids alone
Budesonide is a synthetic corticosteroid with a high topical glucocorticoid activity and a substantial first-pass elimination, reducing systemic absorption; the drug is dosed at 1 mg per cat PO once a day and gradually tapered over 8 to 10 weeks
Probiotics may modulate intestinal microbiota
Consider repeat bouts of pancreatitis and/or other diagnoses (intestinal lymphoma) in cats that fail to have a robust response to medical and dietary therapy or those that have a recrudescence of clinical signs following tapering of the prednisolone therapy
THE TAKE-HOME
Feline cholangitis, pancreatitis, and IBD can manifest with the same clinical signs
Abdominal ultrasound is helpful for evaluating the hepatic parenchyma and biliary tree, pancreatic and surrounding echogenicity, and the intestinal wall layers; however, a normal ultrasound does not rule out intestinal disease, pancreatitis, or cholangitis
Always pursue a comprehensive search to identify secondary causes of hepatic lipidosis
Always assess cobalamin status in cats with a history of anorexia, weight loss, vomiting, and/or diarrhea
Most cats with pancreatitis do not have evidence of abdominal pain
Intestinal disease should be considered in all anorectic cats exhibiting evidence of weight loss, even in the absence of vomiting or diarrhea
IBD and intestinal lymphoma can be segmental in their distribution—the jejunum is the most commonly affected segment of bowel affected with small cell lymphoma
Always procure multiple biopsies from the stomach, duodenum, jejunum, and ileum during laparotomy or laparoscopy even if the bowel appears grossly unremarkable
Enteral feeding devices should be considered in all anorectic cats with triaditis
ALP = alkaline phosphatase, ALT = alanine transaminase, AST = aspartate transaminase, FNA = fine-needle aspiration, IBD = inflammatory bowel disease, SAMe = S-adenosylL-methionine, Spec fPL = feline pancreas-specific lipase
ANN DELLA MAGGIORE, DVM, DACVIM (Internal Medicine), is assistant professor of clinical internal medicine at University of California, Davis. Her clinical interests include endocrinology, infectious and immune-mediated disease, and minimally invasive diagnostic and therapeutic procedures. A UC Davis graduate, Dr. Maggiore completed an internship in small animal medicine and surgery at Veterinary Medical and Surgical Group in Ventura, California, and a residency in small animal internal medicine at UC Davis.
STANLEY L. MARKS, BVSc, PhD, DACVIM (Internal Medicine, Oncology), DACVN, is professor in the department of medicine and epidemiology and director of the Companion Animal Gastrointestinal Laboratory at University of California, Davis. A graduate of University of Pretoria, South Africa, he completed an internship in small animal medicine and surgery at University of Missouri-Columbia, a small animal internal medicine residency program at University of Florida, and an oncology residency program at University of California, Davis, where he earned his doctorate.