Treating Septic Shock

You have asked...
What is septic shock, and how should it be treated?
The expert says…
Shock, a syndrome in which clinical deterioration can occur quickly, requires careful analysis and rapid treatment. A broad definition of shock includes inadequate cellular energy production or the inability of the body to supply cells and tissue with oxygen and nutrients or remove waste products.1-3 Before discussing septic shock, it is necessary to have a general understanding of sepsis, which is the systemic inflammatory response to infection (ie, bacteria, viruses, fungi, parasites).
Related Article: Hypovolemic Shock
Septic shock is defined as acute circulatory failure and persistent arterial hypotension (despite volume resuscitation) associated with sepsis.4 The systemic inflammatory response syndrome(SIRS) can be the result of an infectious insult (sepsis) or a noninfectious insults including trauma, neoplasia, and various sterile inflammatory diseases such as pancreatitis (Table).
Clinical Clues
In early septic shock, common clinical signs in dogs include brick red or injected mucous membranes; a rapid capillary refill time (CRT, <1 second); tachycardia; tachypnea; elevated rectal temperature; and bounding pulses.4 These abnormalities result from peripheral vasodilation. Unlike dogs, cats in the early stages of sepsis and septic shock are likely to exhibit bradycardia and hypothermia, have pale mucous membranes with a prolonged CRT (>2 seconds), and have weak peripheral pulses.4 Underscoring the importance of serial examinations, a patient suffering from sepsis and septic shock will lose its hyperdynamic appearance (in dogs more than in cats) as neurohumoral responses that contribute to physiologic compensation and the early hyperdynamic phase fail (eg, baroreceptor-mediated release of catecholamines; release of counterregulatory hormones, including glucagon, adrenocorticotropic hormone, and cortisol) and the patient decompensates. Examination findings include pale mucous membranes, prolonged CRT (>2 seconds), refractory hypotension, and poor peripheral pulses that can eventually lead to multiple organ failure and death.4
Related Article: The Many Types of Shock
Although not an exhaustive list, based on physical examination, clinical concerns, and POC testing, additional testing may include aerobic and anaerobic blood cultures, urine culture and susceptibility, tracheal wash for cytology and culture and susceptibility, and fine-needle aspirates of affected or concerning organs or masses.
Diagnostics
Diagnostics for a septic patient are heavily swayed by signalment, history, and physical examination findings. Owners should be queried about clinical signs involving organ systems commonly associated with infection (oral cavity, urogenital system, skin and subcutaneous tissues, abdomen and peritoneal cavity, respiratory and GI tracts). For example, a febrile intact female dog with purulent vaginal discharge might prompt the clinician to evaluate for the presence of pyometra. Other signalment and historical information prompts the clinician to evaluate for other common causes of sepsis (peritonitis, prostatitis, prostatic abscess, pyelonephritis, pneumonia, pyothorax).
Hospitalized patients should also be evaluated for nosocomial infections, including infection as a result of IV and urinary catheters. Translocation of bacteria from a diseased or inflamed GI tract is another possible source of infection. Regardless of underlying cause, the clinician should identify the need for immediate, point-of-care (POC), and second- or third-tier diagnostics.
POC diagnostics when evaluating septic shock may include a CBC (including blood smear to assess WBCs for toxic changes and for pathogens associated with leukocytes or erythrocytes), chemistry panel, urinalysis, coagulation parameters, serum lactate concentration, and blood pressure measurement. Imaging (thoracic and abdominal radiographs, abdominal ultrasound) may also be indicated. Feline POC infectious disease testing may include evaluation for feline immunodeficiency virus, feline leukemia virus, and Dirofilaria immitis (heartworm) infection; canine POC tests include evaluation for D immitis, Borrelia burgdorferi, Ehrlichia spp, and Anaplasma spp infection.
Additional testing may include aerobic and anaerobic blood cultures, urine culture and susceptibility, tracheal wash for cytology and culture and susceptibility, and fine-needle aspirates of affected or concerning organs or masses. Biochemical markers for sepsis have also been investigated, including procalcitonin and C-reactive protein (CRP) in dogs. At this time, while CRP likely has some prognostic utility, studies have not been performed to validate this as a bedside or rapid test.<sup5–8sup>
Treatment
Fluid Therapy
Because patients in septic shock are in acute circulatory failure with persistent arterial hypotension despite volume resuscitation,4 IV fluid therapy should have already been aggressively administered without achieving end-point goals of resuscitation (mean arterial pressure, >65 mm Hg; urine output, >0.5 mL/kg/h; and maintaining central venous oxygen saturation, >70%).9,10 Fluid therapy options include isotonic crystalloids (lactated Ringer’s solution, Normosol-R [hospira.com], Plasma-Lyte A [abbottanimalhealth.com], 0.9% saline); hypertonic crystalloids (ie, hypertonic saline 7.2%); synthetic colloid solutions (ie, hetastarch, dextrans); recombinant human products such as human serum albumin (5% or 25%); polymerized stromal bovine hemoglobin solutions (eg, Oxyglobin, [hbo2therapeutics.com]); and transfusion therapy including plasma products for coagulation and albumin support.
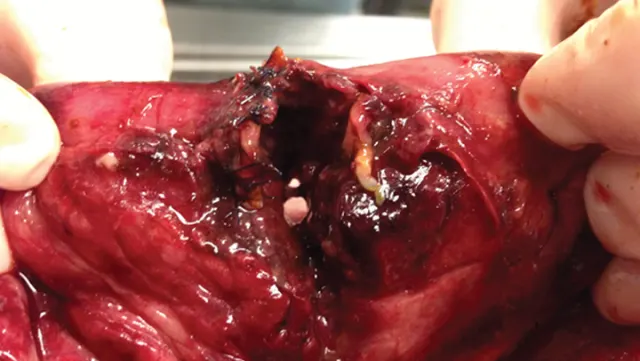
An example of dehiscence of a resection and anastomosis of a dog following a foreign body surgery.
Fluid and administration rates for hypovolemic and hypotensive patients remain controversial. The author recommends the initial use of an isotonic crystalloid with a shock rate of 90 mL/kg in dogs and 45–60 mL/kg in cats. Typically, the entire shock is not given as a single bolus, but rather an aliquot (one-third to one-fourth) followed by patient reassessment, with the goal of hemodynamic stability. An additional crystalloid fluid bolus can be considered if response to therapy is inadequate. Conversely, a synthetic colloid such as hetastarch can be considered (1–3 mL/kg bolus for cats and 3–5 mL/kg for dogs), followed by patient reassessment.
When intravascular volume is adequate (normal CVP 5–10 cm H2O) but the patient remains hypotensive, administration of positive inotropes or vasopressors should be considered. If CVP evaluation is not possible, monitor the patient’s body weight, mentation, skin turgor, pulse rate and quality, respiratory rate and effort, urine output, urine specific gravity, mucous membrane color, and CRT. Perform serial lung auscultation to detect increased bronchovesicular sounds or crackles. Dopamine, dobutamine, norepinephrine, and/or vasopressin are options to consider.
Related Article: Bacterial Septic Peritonitis
Infection Control
Antibiotic therapy is essential in sepsis treatment and should be instituted as early as possible following diagnosis. Ideally, collecting samples for diagnostic evaluation (urine, blood, and cerebrospinal fluid cultures) should be performed before the use of antibiotics. Antibiotic therapy should not be delayed if diagnostic samples cannot be obtained in a timely fashion. Whereas antibiotic therapy is ideally based on the results of an aerobic and anaerobic culture and susceptibility, gram-negative enteric organisms such as Escherichia coli are most commonly found in small animal patients with sepsis.11,12 Initial empirical broad-spectrum IV antibiotic therapy should have activity against these common pathogens:
Ampicillin (22 mg/kg IV q8h) and enrofloxacin (dogs, 5–20 mg/kg IV q24h; cats, 5 mg/kg IV q24h)
Ampicillin (22 mg/kg IV q8h) and cefoxitin (15–30 mg/kg IV q4–6h)
Clindamycin (8–10 mg/kg IV q12h) and enrofloxacin (dogs, 5–20 mg/kg IV q24h; cats, 5 mg/kg IV q24h)
Ticarcillin and clavulanic acid (50 mg/kg IV q6h) and enrofloxacin (dogs, 5–20 mg/kg IV q24h; cats, 5 mg/kg IV q24h)13,14
Organ Support
With hypotension and hypoperfusion, blood is shunted away from organs such as the intestines to more vital organs such as the heart, brain, and kidneys. Possible complications include ileus, gastric malperfusion and ulceration, and intestinal mucosal injury that can lead to bacterial and endotoxin translocation.15,16 Clinicians should, therefore, consider GI-protective strategies and enteral nutrition. GI prophylaxis may include H2-receptor antagonists, proton pump inhibitors, and sucralfate. Prokinetic agents such as metoclopramide may also help with GI motility.
Adequate nutrition is also vital to the survival of patients with sepsis. Enteral nutrition has been shown to improve tissue healing, maintain protein levels, and provide substrates for energy production and normal homeostatic mechanisms.17 Enteral nutrition can be administered through nasoesophageal, esophagostomy, gastrostomy, or jejunostomy tubes.
Oxygen therapy is also indicated for any patient with evidence of hypoxemia. Any patient with evidence of hypoxemia (PaO2 <80 mm Hg or SpO2 <95%) should receive supplemental oxygen.
In addition, septic patients have a risk for hypoglycemia as sepsis is associated with increased peripheral glucose utilization caused by a hypermetabolic state. Septic patients with hypoglycemia require supplementation with IV dextrose. Hypoglycemic patients in crisis should receive a dextrose bolus (0.25–0.5 gm/kg IV) followed by a CRI in their fluids (2.5%–5%). Before administration as a bolus, dextrose should be diluted to 10% concentration and delivered through an IV peripheral catheter as it is hypertonic and can cause vasculitis and red cell lysis. To achieve a 10% solution, 50% dextrose should be diluted 1:4 with sterile water. For use as a 5% CRI, 100 mL of 50% dextrose is added to 1 L of fluids to make a 5% solution.
Conclusion
Sepsis and septic shock should be carefully evaluated and quickly treated as they often result in severe cardiovascular abnormalities, multiple organ failure, and death. Aggressive fluid resuscitation to promote adequate tissue perfusion alone may be unsuccessful; additional therapy such as vasopressor support is often needed. The clinician should also focus on infection control and organ-protective strategies to reduce morbidity and mortality. Despite aggressive therapy and early intervention, mortality rates range from 20%–68%.17-21
GARRET E. PACHTINGER, VMD, DACVECC, is an associate at the Veterinary Specialty and Emergency Center in Levittown, Pennsylvania. He is actively involved in the American College of Veterinary Emergency and Critical Care. Dr. Pachtinger’s research and clinical interests include toxicology, respiratory diseases, mechanical ventilation, renal disease, and fluid therapy in critically ill patients. He has published numerous scientific articles and book chapters, and he lectures nationally and internationally, including at the International Veterinary Emergency & Critical Care Symposium and the NAVC Conference.