Cystine Urolithiasis
Gregory F. Grauer, DVM, MS, DACVIM (SAIM), Kansas State University

How do I manage cystine urolithiasis?
Cystine is a nonessential sulfur-containing amino acid made up of 2 cysteine molecules joined by a disulfide bond (Figure 1). Cysteine is found in most high-protein foods, including pork, poultry, eggs, and dairy products, as well as oats and wheat germ. Cystine is absorbed by the small intestine, freely filtered by the glomerulus, and then reabsorbed by an active process in the proximal convoluted tubule.
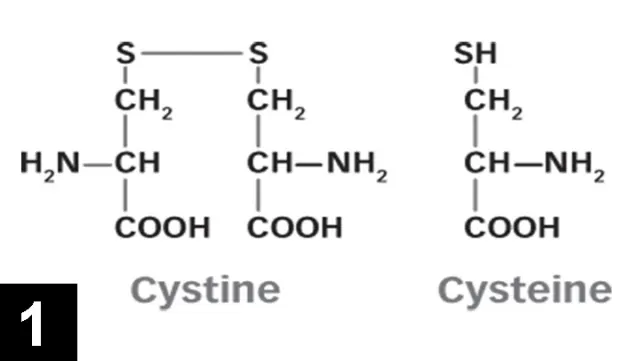
FIGURE 1 Structure of cystine and cysteine
Decreased tubular reabsorption of cystine results in cystinuria. Cystinuria (>75–125 mg cystine/g creatinine) is a predisposing and required factor for—but not the sole cause of—cystine urolith formation; not all dogs with cystinuria form cystine uroliths or even have cystine crystals in their urine; the exact mechanism of cystine urolith formation is unknown. Cystine is most soluble in alkaline solutions, hence cystine uroliths usually form in acidic urine. Cystinuria can be detected with a cyanide–nitroprusside test (available at the University of Pennsylvania), but ampicillin and sulfur-containing drugs in the urine may cause false-positive results.
Cystine crystals are flat, colorless, and hexagonal. The sides of the hexagon may or may not be of equal lengths, and the crystals tend to aggregate in urine sediment, resulting in a layered appearance (Figure 2). Cystine uroliths have intermediate radiodensity; they are typically less radiodense than calcium oxalate and struvite uroliths but more so than ammonium urate uroliths (Figures 3 and 4). In some cases, contrast urethrocystography or ultrasonography may be necessary to visualize uroliths. Cystine uroliths are usually smooth and spherical and range in size from less than 1 mm to greater than 3 cm in diameter. Affected dogs frequently present with multiple cystine uroliths (Figure 5). Secondary urinary tract infections are uncommon.

FIGURE 2 Cystine crystals in urine sediment from a maned wolf. Image courtesy Dr. Lisa Pohlman
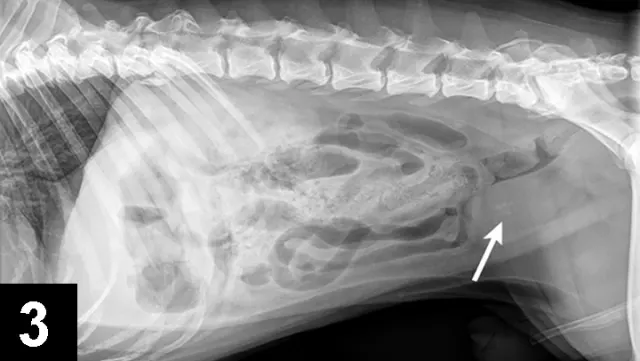
FIGURE 3 Lateral radiograph of a 5-year-old, castrated English bulldog with multiple cystine uroliths in the kidneys, bladder, and urethra (arrow).
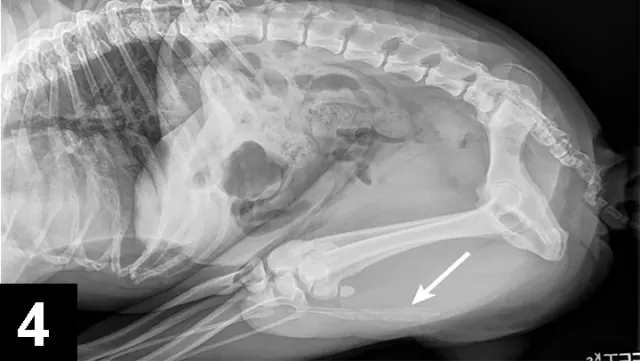
FIGURE 4 Tucked lateral radiograph of the dog from Figure 2 showing additional calcium oxalate urethroliths (arrow).

FIGURE 5 100% cystine uroliths from the dog in Figures 2 and 3 (scale, 1 division = 1 mm).
Dachshund, mastiff, Chihuahua, Welsh corgi, bullmastiff, Scottish deerhound, basset hound, Irish terrier, English bulldog, and Newfoundland breeds are predisposed to forming cystine uroliths. Owing to the greater popularity of these breeds in Europe than in the U.S., the prevalence of uroliths varies geographically (>30% in some European studies vs. 1%–3% in the U.S.). The mean age of dogs with cystine uroliths is 4-6 years. Male dogs (98%) are affected to a much greater degree than females (2%), and most cystine uroliths (98%) are found in the lower urinary tract (bladder and urethra). Newfoundlands appear to be an exception as cystine uroliths form in younger (<1 year of age) males and females and can also be found in the kidney. Maned wolves from South America have also been shown to have a high incidence of cystinuria and are predisposed to forming cystine uroliths.
Only 0.15% of the feline uroliths analyzed at the GV Ling Urinary Stone Analysis Laboratory at the University of California, Davis, are cystine. Cystine uroliths in cats are found in male and female domestic shorthair and Siamese breeds over a wide age range (4 months–12 years). As is true in dogs, cystine uroliths form in cats with cystinuria.
Pathophysiology of Cystine Uroliths
Cystinuria is abnormal and associated with a defect in tubular reabsorption of cystine. This tubular defect may also extend to other dibasic amino acids, including ornithine, lysine, and arginine (with cystine, collectively known as COLA); however, plasma concentrations of these amino acids are typically normal in affected dogs unless dietary protein is severely restricted. Were it not for the relative insolubility of cystine in urine and the tendency of cystinuric dogs to form uroliths, cystinuria would have no medical consequence.
Cystinuria is inherited as an autosomal recessive trait in Newfoundlands2 (and likely Labrador retrievers7) and is associated with a mutation in the renal basic amino acid transporter (rBAT). Testing for this mutation is available, and breeding management in response to such testing may be responsible for the recently observed decrease in cystine uroliths in the U.S. The rBAT gene mutation has not been found in other breeds with cystinuria. The magnitude of the tubular reabsorptive defect in Newfoundlands is more severe than with other breeds and likely explains why cystine uroliths are observed in younger Newfoundlands and the kidneys. Recurrence for cystine uroliths after dissolution or removal is high in all breeds but tends to be highest in Newfoundlands.
Urine cystine concentrations should be <200 µmol/g creatinine and/or COLA is <700 µmol/g creatinine to prevent recurrence. Urine cystine and/or COLA concentrations can be measured at the School of Veterinary Medicine at University of Pennsylvania, Section of Medical Genetics.
Dissolution of Cystine Uroliths
Cystine uroliths can be removed surgically or mechanically (eg, voiding urohydropropulsion, catheter or stone basket retrieval, lithotripsy, dissolved through medical management). High recurrence rates makes medical management to prevent recurrence necessary, even if uroliths are removed. Before initiating dissolution treatment, cystine urethroliths must be retropulsed into the urinary bladder.
Medical dissolution protocols involve both dietary and drug components. The foundation of medical dissolution is to feed alkalinizing, high-moisture (ideally canned) diets that are low in protein (and therefore amino acids) and salt (natriuresis caused by increased dietary salt results in increased cystine excretion).2 In addition to dietary changes, N-(2-mercaptopropionyl)-glycine (2-MPG; Thiola, Mission Pharmacal) is administered at 15 mg/kg PO q12h. 2-MPG binds with cysteine in the urine to form a complex that is more soluble than cystine. Finally, urine alkalinizing agents like potassium citrate may be necessary, if diet alone does not maintain a urine pH in the 7 to 7.5 range. Urine should be monitored at least monthly for pH and specific gravity (ideal, <1.020). Imaging to assess urolith size and location is recommended every 4 weeks, and medical management should continue for 4 weeks after uroliths are no longer visible; cystine levels should be monitored during treatment in recurrent cases.
Prevention of Cystine Uroliths
Preventing cystine urolith recurrence may be possible with diet alone in some patients. Dietary considerations are similar to those for dissolution (Hill’s Prescription Diet u/d Canine, Royal Canin Veterinary Diet Canine Urinary UC Low Purine, Royal Canin Veterinary Diet Canine Vegetarian, and Purina Veterinary Diet HA Hypo-allergenic Canine Formula). If necessary, 2-MPG (15 mg/kg PO q12h) and potassium citrate (0.5 mEq/kg/day PO [starting dose]) can be added to the prevention regimen. Urinalysis and imaging studies should be repeated every 3-6 months or when signs of lower urinary tract infection are present (eg, inappropriate urination, stranguria/dysuria, hematuria).